Process for preparing rifaximin
A preparation process and a rifaximin technology, applied in the field of rifaximin synthesis process, can solve the problems of high consumption of raw materials and energy, low production efficiency, large human injury, etc., so as to reduce treatment costs, protect the environment and save energy. energy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
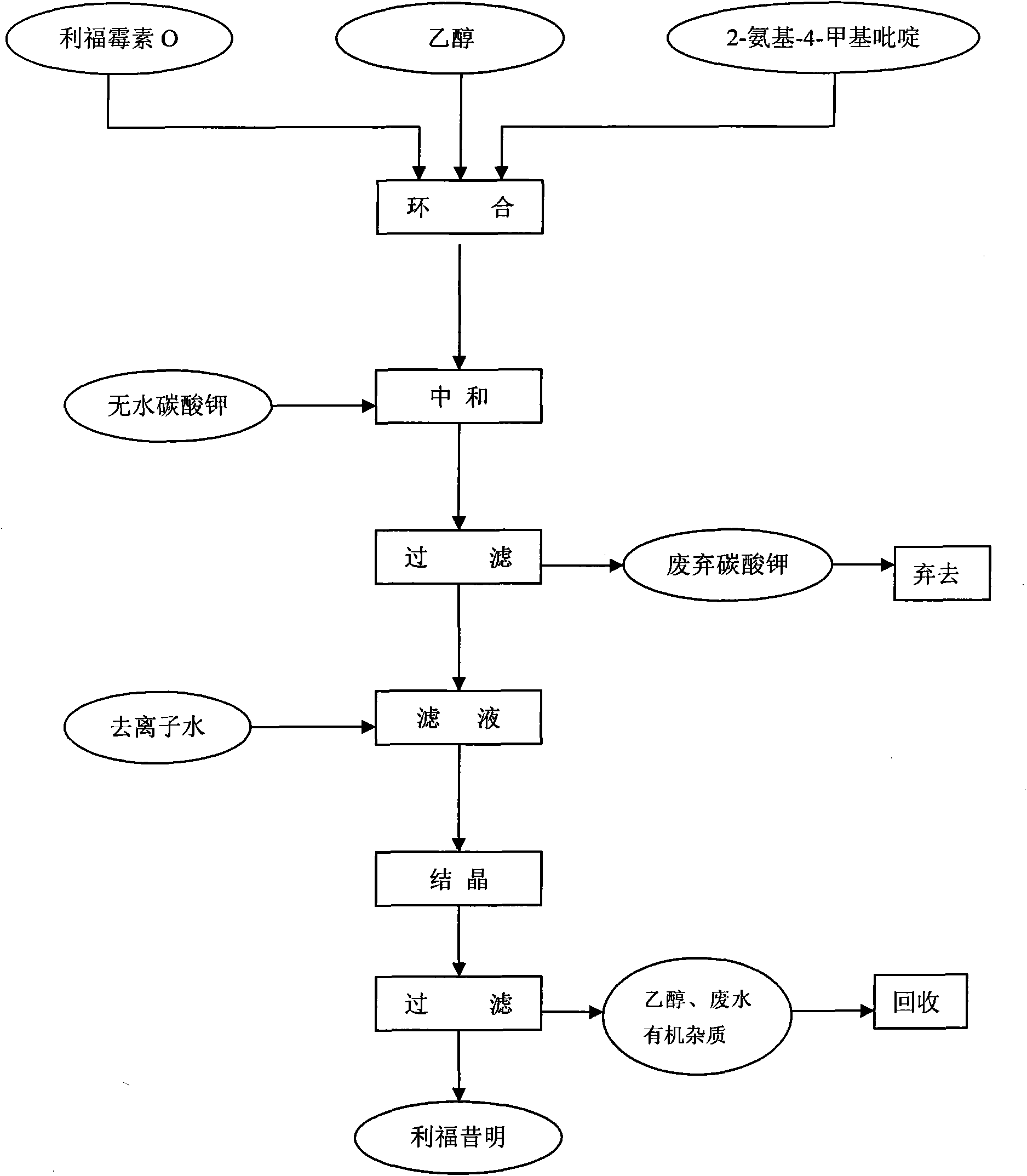
[0031] figure 1 For the preparation process flow chart of the present invention, in a 1000ml three-necked round-bottomed flask equipped with an electric stirrer, a thermometer and a reflux device, 400ml of ethanol was added, and 75.4g (0.1mol) of rifamycin O and 27g ( 0.25mol) 2-amino-4-methylpyridine, the temperature was raised to 40°C, and the reaction was stirred for 24 hours. After the reaction is completed, add 12.5g (0.09mol) of anhydrous potassium carbonate, stir for 30 minutes, filter, add 170ml of deionized water to the filtrate, crystallize, filter, and dry the filter cake to obtain 70.1g of rifaximin finished product, melting point: 200 ~205°C (decomposition), yield 89%.
Embodiment 2
[0033] The reaction vessel was the same as in Example 1, 500 ml of ethanol was added, 75.4 g (0.1 mol) of rifamycin O and 21.6 g (0.2 mol) of 2-amino-4-picoline were added successively under stirring, and the temperature was raised to 45 ° C and stirred. The reaction was carried out for 20 hours. After the reaction was completed, 13.8 g (0.1 mol) of anhydrous potassium carbonate was added, stirred for 30 minutes, filtered, and 180 ml of deionized water was added to the filtrate for crystallization to obtain 68.5 g of finished rifaximin with a yield of 87%.
Embodiment 3
[0035] The preparation process is the same as in Example 1, except that the addition of 2-amino-4-methylpyridine is 32.4g (0.3mol), and after the reaction is completed, 15.2g (0.11mol) of anhydrous potassium carbonate is added, and finally rife is obtained. The finished product of Ximing was 69.3 g, and the yield was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com