Coding beta-galactosidase gene and expression and application thereof
A galactosidase and gene technology, applied in the field of enzyme genetic engineering, can solve problems such as unclear mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
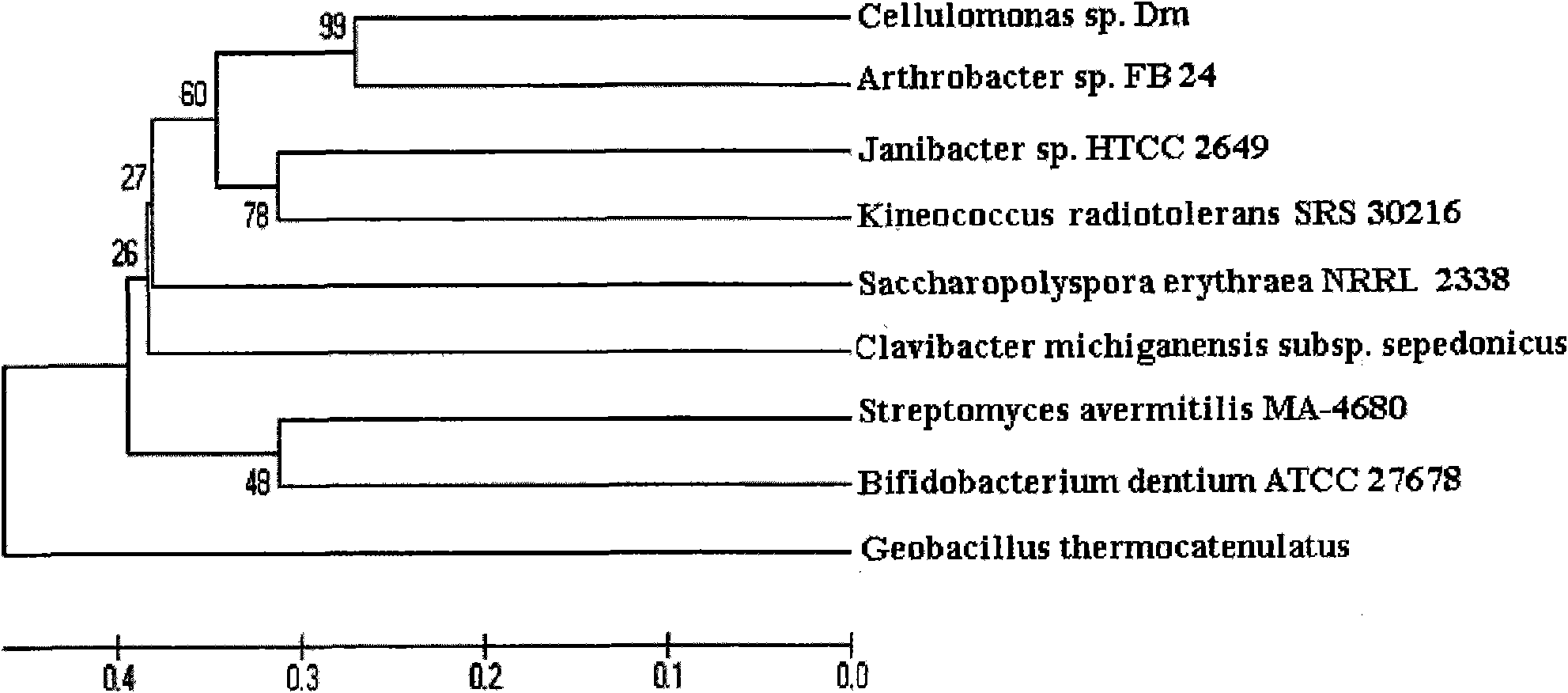
[0051] Example 1 Screening, isolation and identification of Cellulomonas producing β-galactosidase
[0052] 1.1 Materials
[0053] 1.1.1 Sample
[0054] A total of 12 kinds of soil samples were collected from Xinjiang.
[0055] 1.1.2 Tool enzymes and reagents
[0056] Pfu high-fidelity DNA polymerase was purchased from Qihuasheng Biological Company; restriction endonuclease and T4 DNA ligase were purchased from TaKaRa and NEB Company; RNase was purchased from Beijing Huamei Company; DNA purification kit was purchased from Shanghai Shenneng Gaming Company; The substance o-nitrophenol-β-D-galactoside (ONPG) was purchased from Pierce; 5-bromo-4-chloro-3-indole-D-galactoside (X-gal) was purchased from TaKaRa; dNTP was purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd.; yeast extract and peptone were purchased from Oxford; dimethylformamide (DMF) was purchased from Shanghai Bioengineering Co., Ltd.; other reagents were of domestic analytical grade.
[0057] 1.1.3...
Embodiment 2
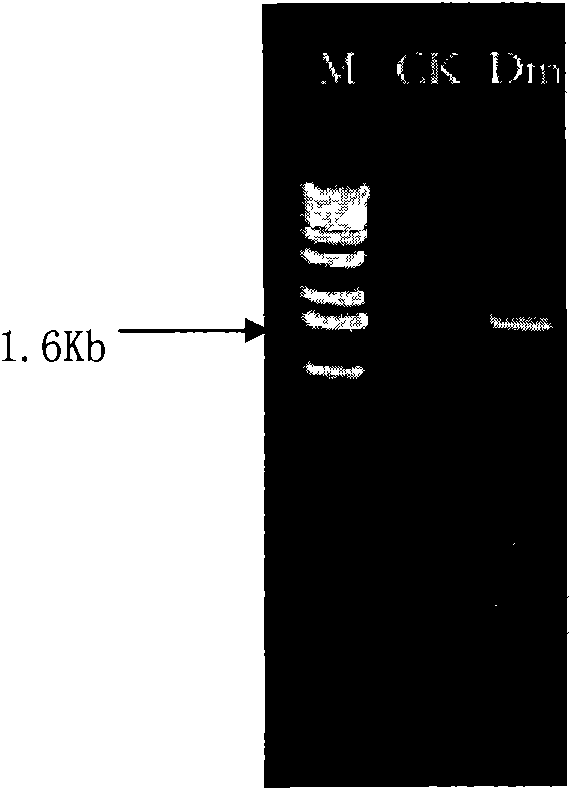
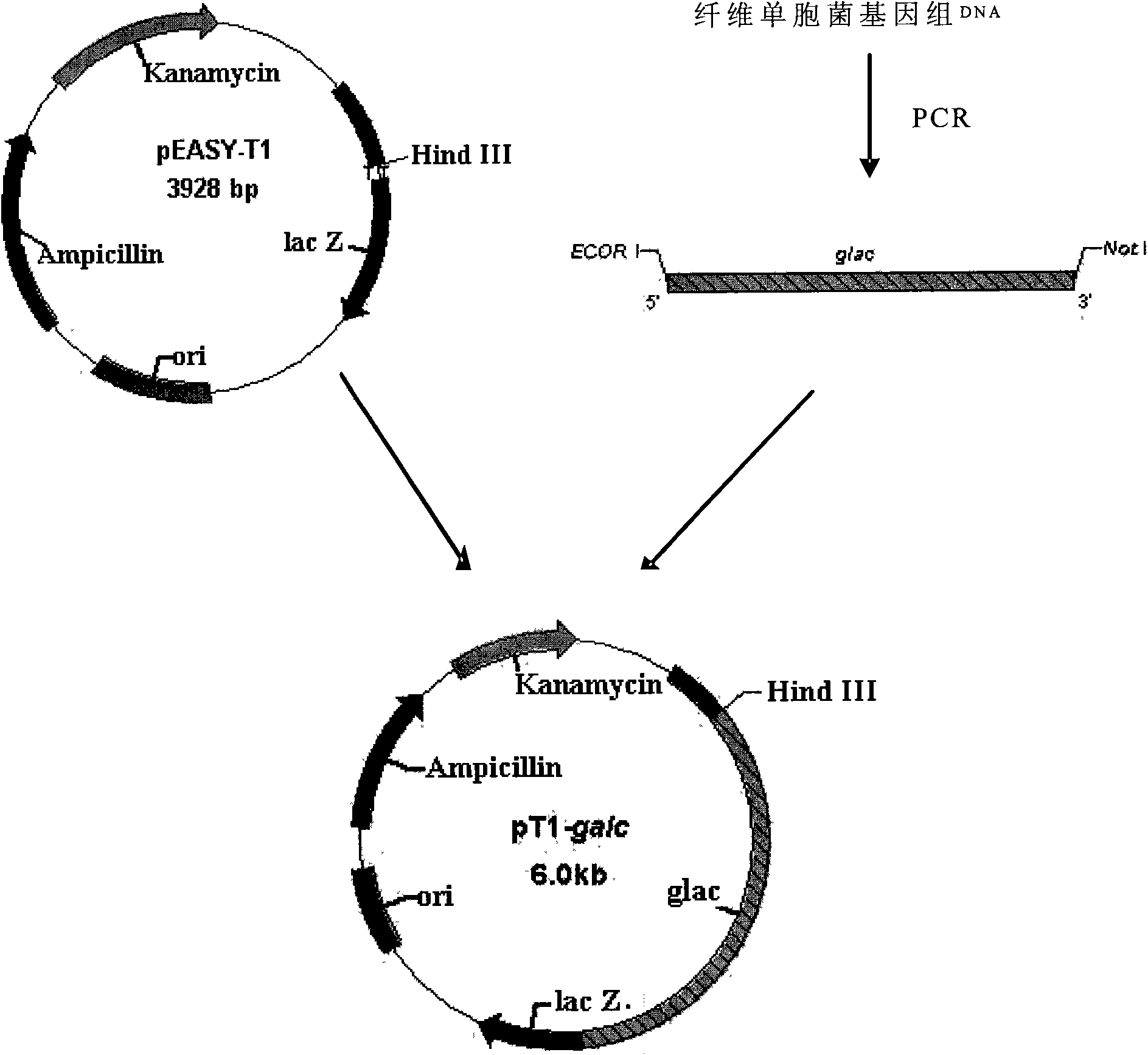
[0076] Isolation and cloning of embodiment 2β-galactosidase gene
[0077] 2.1 Materials
[0078] 2.1.1 Strains and plasmids
[0079] Cellulomonas Dm (Cellulomonas sp.) is obtained by screening in Example 1;
[0080] The pEASY-T1 cloning vector was purchased from Beijing Quanshijin Biological Company;
[0081] Top 10 Escherichia coli competent cells were purchased from Beijing Quanshijin Biological Company.
[0082] 2.1.2 Tool enzymes and reagents
[0083] LA TAq DNA polymerase was purchased from Takara Company;
[0084] Phusion ultra-fidelity PCR kit was purchased from NEB Company;
[0085] All other chemical reagents were of domestic analytical grade.
[0086] 2.1.3 Culture medium and related solution preparation
[0087] Lactose separation medium: 2.0% lactose, 0.5% yeast extract, 1.0% peptone, 0.5% NaCI, sterilized at 115°C for 15 minutes;
[0088] TAE (50×): 242g Tris base, 57.1ml glacial acetic acid, 100ml 0.5mol / L EDTA (pH8.0), dilute to 1L with sterile water.
[...
Embodiment 3
[0256] Expression of embodiment 3β-galactosidase gene galc
[0257] 3.1 Materials
[0258] 3.1.1 Strains and plasmids
[0259] Escherichia coli TOP10 and Escherichia coli BL21 (DE3) were purchased from Beijing Quanshijin Biological Company;
[0260] The pET-30a(+) vector was purchased from Novagen.
[0261] 3.1.2 Tool enzymes and reagents
[0262] IPTG was purchased from Promega;
[0263] Protein molecular weight standard DM101 and DR101 were purchased from Beijing Quanshijin Biological Company;
[0264] Acrylamide, N,N′-methylene acrylamide, and agarose were purchased from Sigma;
[0265] Affinity chromatography resin Ni-NTA Agarose was purchased from Qiagen;
[0266] Others are domestic analytical reagents.
[0267] 3.1.3 Configuration of medium and related solutions
[0268] Protein loading buffer (2×): 100mmol / L Tris-HCl (pH6.8), 200mmol / L dithiothreitol (DTT), 4% SDS, 0.2% bromophenol blue, 10% glycerol.
[0269] 30% acrylamide solution: 29g of acrylamide, 1g of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com