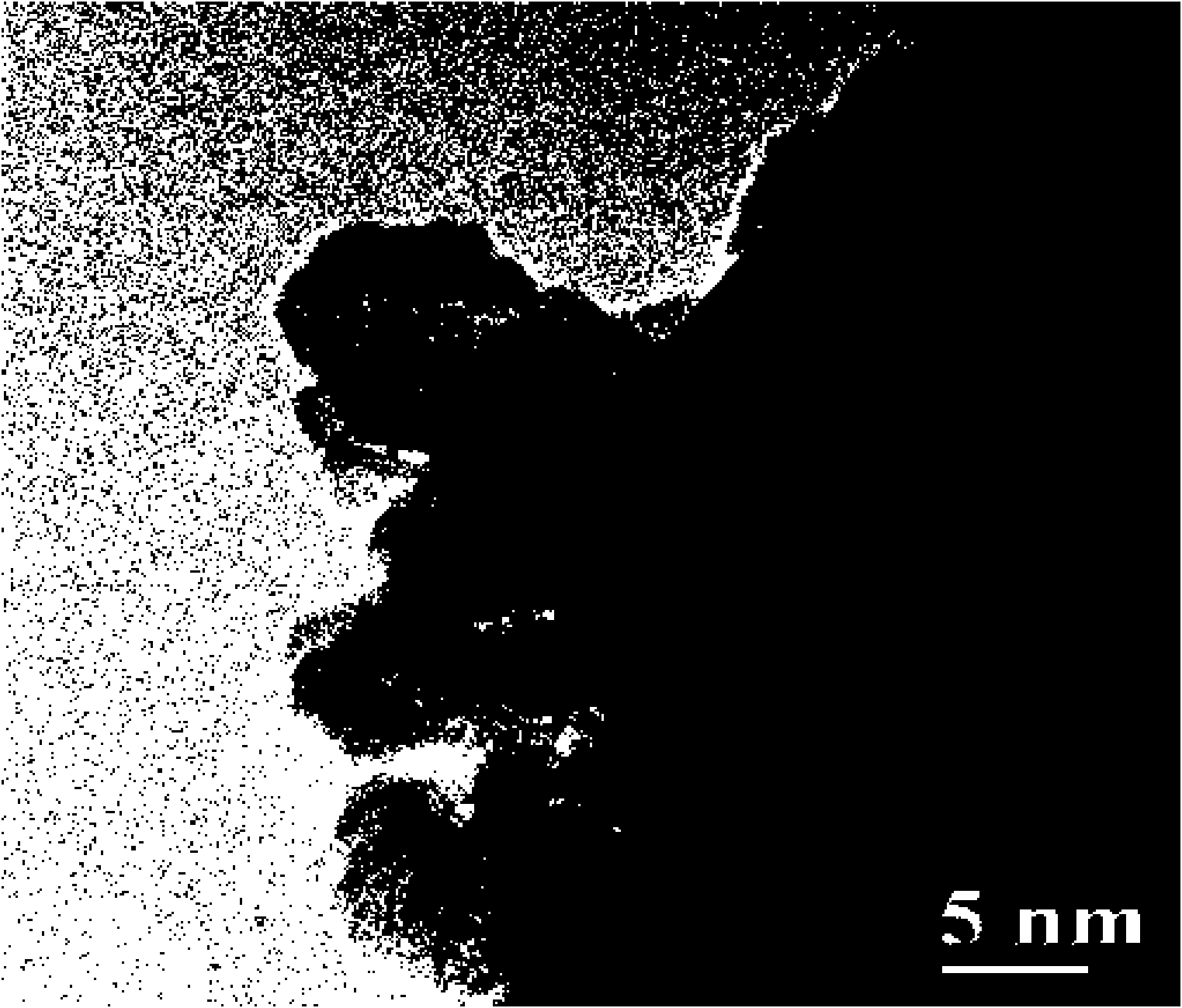Catalyst for hydrogen production by self-heating reforming of methanol and preparation method thereof
An autothermal reforming and catalyst technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. Complex problems such as improved catalytic activity and stability, good heat and mass transfer performance, and high catalyst utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
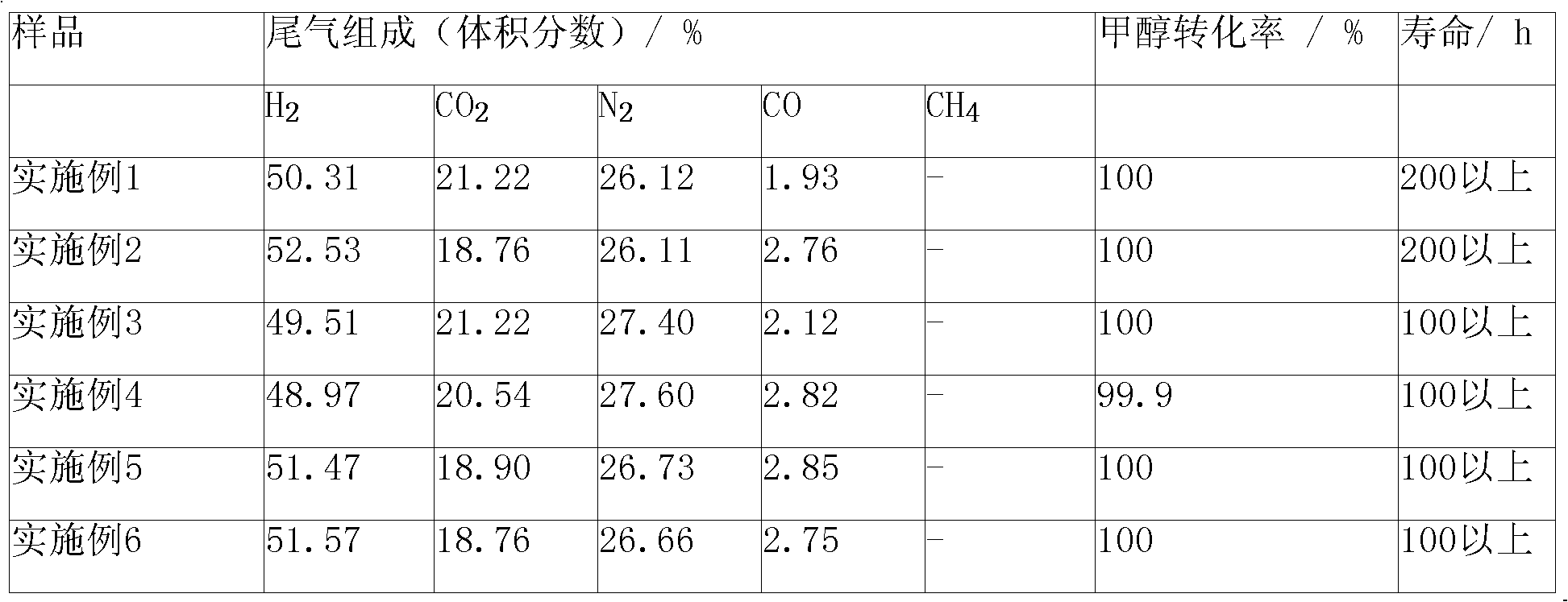
Embodiment 1
[0037] ZnO-Cr 2 o 3 / C 0.30 Zr 0.15 Y 0.025 La 0.025 al 0.5 o 2 Monolithic catalyst, the preparation method is as follows:
[0038] (1) Ce(NO with a molar ratio of 6:3:10 3 ) 3 ·6H 2 O, Zr(NO 3 ) 3 ·3H 2 O, Al(NO 3 ) 3 9H 2 O and 0.5% La(NO 3 ) 3 ·6H 2 O, Y (NO 3 ) 3 ·6H 2 O is dissolved in metered deionized water, and the two are configured into a solution with a concentration of 0.5mol / L; the industrial ammonia water with a concentration of 25% is diluted twice as a precipitant, and the above salt solution is added dropwise to the above precipitation under continuous stirring In the solution, control the pH=9. After the precipitation is complete, age at room temperature for 12 hours, wash twice with deionized water, replace the water in the obtained precipitate with absolute ethanol, supercritically dry, and roast at 800 ° C for 6 hours to obtain Ce 0.30 Zr 0.15 Y 0.025 La 0.025 al 0.5 o 2 Nano-cerium-zirconium-aluminum composite oxide support, cha...
Embodiment 2
[0042] ZnO-Cr 2 o 3 / C 0.193 Zr 0.032 Y 0.1 al 0.675 o 2 Monolithic catalyst, the preparation method is as follows:
[0043] (1) Ce(NO with a molar ratio of 6:1:21 3 ) 3 ·6H 2 O, Zr(NO 3 ) 3 ·3H 2 O, Al(NO 3 ) 3 9H 2 O and Y (NO 3 ) 3 ·6H 2 O is dissolved in metered deionized water, and the two are configured into a solution with a concentration of 0.5mol / L; the industrial ammonia water with a concentration of 25% is diluted twice as a precipitant, and the above salt solution is added dropwise to the above precipitation under continuous stirring In the solution, control the pH=8. After the precipitation is complete, age at room temperature for 12 hours, wash twice with deionized water, replace the water in the obtained precipitate with absolute ethanol, supercritically dry, and roast at 500 ° C for 5 hours to obtain Ce 0.193 Zr 0.032 Y 0.1 al 0.675 o 2 Nano-cerium-zirconium-aluminum composite oxide carrier, characterized by TEM, the particle diameter of t...
Embodiment 3
[0047] ZnO-Cr 2 o 3 / Pr 2 o 6 -Ce 0.102 Zr 0.611 Y 0.05 al 0.237 o 2 Monolithic catalyst, the preparation method is as follows:
[0048] (1) Ce(NO with a molar ratio of 3:18:7 3 ) 3 ·6H2 O, Zr(NO 3 ) 3 ·3H 2 O, Al(NO 3 ) 3 9H 2 O and Y (NO 3 ) 3 ·6H 2 O and 0.5% by weight of Pr(NO 3 ) 3 ·6H 2 O is dissolved in metered deionized water to form a solution with a concentration of 0.5mol / L; dilute industrial ammonia water with a concentration of 25% twice as a precipitant, and add the above salt solution dropwise to the precipitant under continuous stirring , control the pH=10, after the precipitation is complete, age at room temperature for 12 h, wash twice with deionized water, replace the water in the obtained precipitate with absolute ethanol, supercritically dry, and roast at 650 ° C for 2 h to obtain Pr 2 o 6 -Ce 0.102 Zr 0.611 Y 0.05 Al 0.237 o 2 Nano-cerium-zirconium-aluminum composite oxide carrier, characterized by TEM, the particle diameter of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com