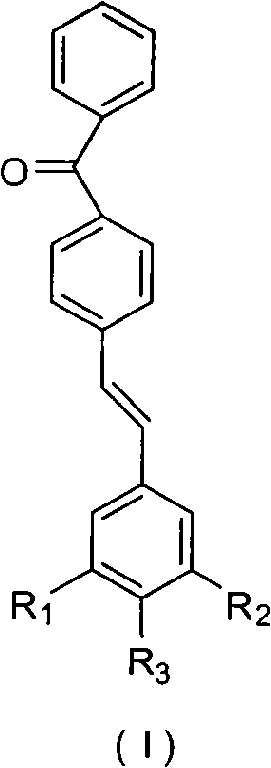
Benzophenone-containing conjugated stilbene dye and synthesis and application thereof
A technology of benzophenone stilbene and benzophenone diethyl phosphonate, applied in the field of visible light initiators, can solve the problems of low initiation efficiency, short absorption wavelength and the like, and achieves suitable yield and absorption wavelength. The effect of wide, convenient source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
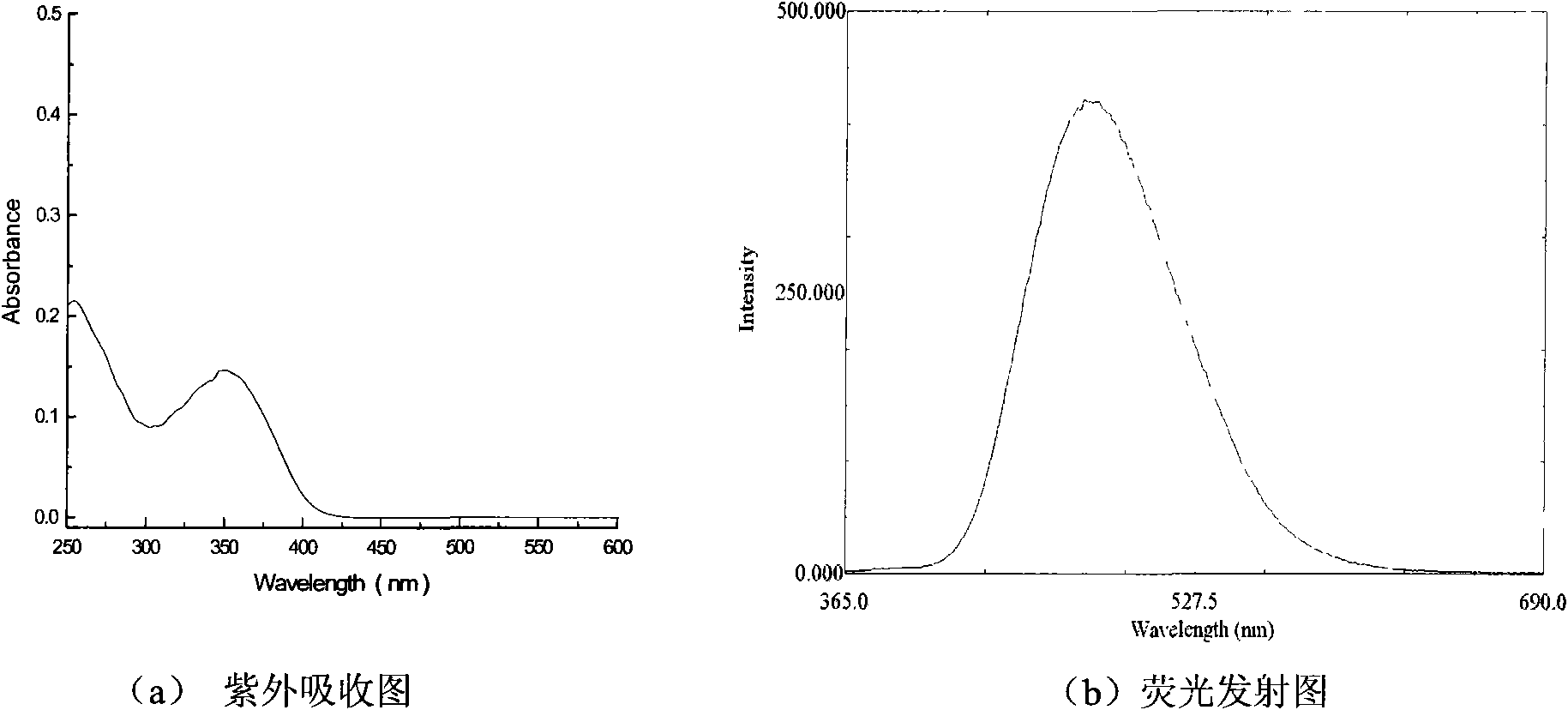
[0045] (4-(3,4-Dihydroxystyryl)phenyl)(phenyl)methanone
[0046] The synthesis proceeds in three steps:
[0047] (1) Synthesis of 4-bromomethylbenzophenone
[0048] 1.0g 4-methylbenzophenone is mixed with 0.91g N-bromosuccinimide (NBS) (molar ratio 1: 1) in a three-necked ground-necked flask, and a condensing device is added. 4 Reflux for 6 hours, filter, recrystallize the solid in benzene / cycloethane, filter to obtain crystals, the yield is 55%, and set aside;
[0049] (2) Synthesis of 4-benzophenone-phosphonic acid diethyl ester
[0050] The ratio of 2.75g 4-bromomethylbenzophenone synthesized in the first step and 16.6g triethyl phosphinate (molar ratio 1:10) was reacted at 145°C for some time, cooled, and excess phosphinate was removed under reduced pressure. Triethyl phosphonate was obtained to obtain diethyl 4-benzophenonyl phosphonate, without further purification, the yield was 86%, and it was set aside;
[0051] (3) Synthesis of (4-(3,4-dihydroxystyryl) phenyl) (p...
Embodiment 2
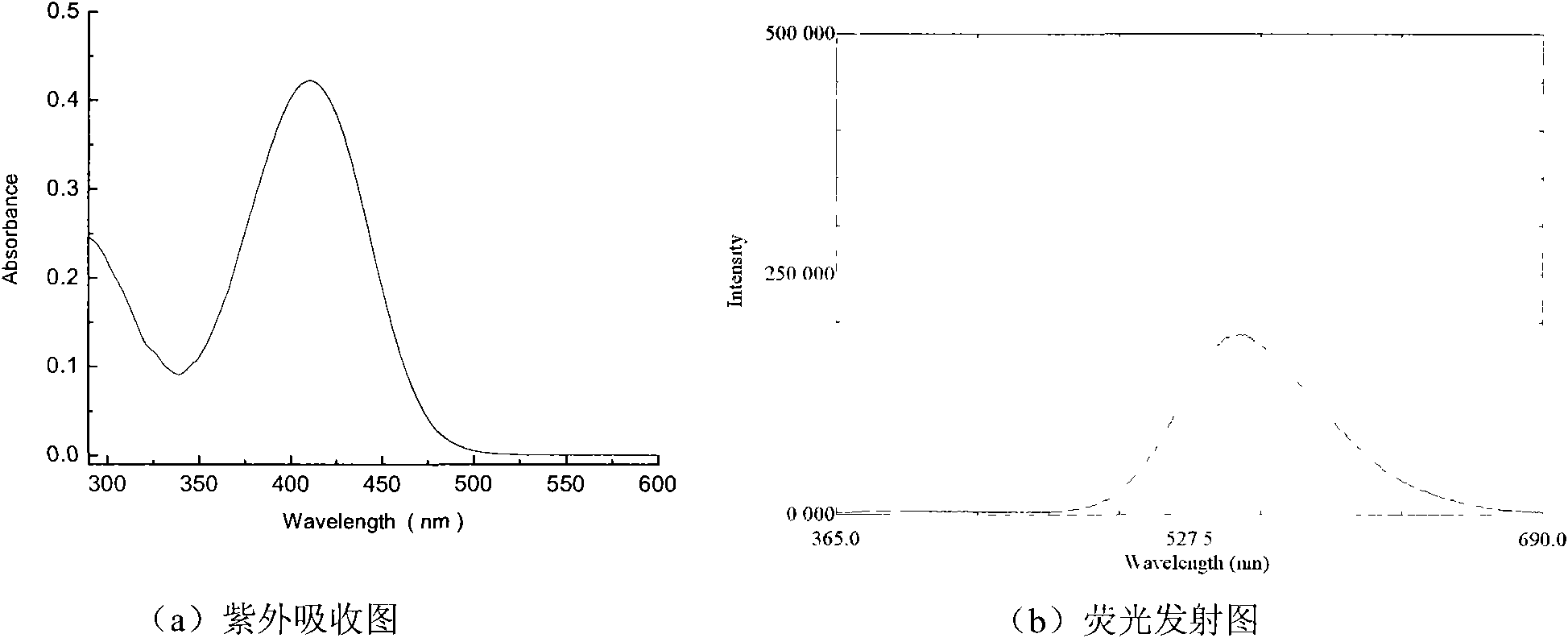
[0054] Synthesis of (4-(4-(diethylamino)styryl)phenyl)(phenyl)methanone
[0055] The synthesis proceeds in three steps:
[0056] (1) Synthesis of 4-bromomethylbenzophenone
[0057] Carry out in the first step according to embodiment 1;
[0058] (2) Synthesis of 4-benzophenone-phosphonic acid diethyl ester
[0059] Synthesis is carried out by the second step in the implementation case 1;
[0060] (3) Synthesis of (4-(4-(diethylamino)styryl)phenyl)(phenyl)methanone
[0061] Add 1.6 g of diethyl 4-benzophenonylphosphonate synthesized in the second step into a three-necked flask, add dry tetrahydrofuran, add a basic catalyst in an ice bath, stir magnetically for half an hour, and remove the ice bath, add 0.85g of N, N-diethylbenzaldehyde, and then stir the reaction at room temperature for 24 hours. The solvent is evaporated to dryness in a rotary evaporator, and the remaining mixture is dissolved in chloroform, washed three times with primary distilled water, and washed with c...
Embodiment 3
[0063] Synthesis of (4-(4-nitrostyryl)phenyl)(phenyl)methanone
[0064] The synthesis proceeds in three steps:
[0065] (1) Synthesis of 4-bromomethylbenzophenone
[0066] Carry out in the first step according to embodiment 1;
[0067] (2) Synthesis of 4-benzophenone-phosphonic acid diethyl ester
[0068] Synthesis is carried out by the second step in the implementation case 1;
[0069] (3) Synthesis of (4-(4-nitrostyryl) phenyl) (phenyl) ketone
[0070] Add 1.7 g of diethyl 4-benzophenonylphosphonate synthesized in the second step into a three-necked flask, add dry tetrahydrofuran, add a basic catalyst in an ice bath, stir magnetically for half an hour, and remove the ice bath, add 0.77g of N,N-diethylbenzaldehyde, and then stir the reaction at room temperature for 24 hours, the solvent is evaporated to dryness in a rotary evaporator, and the remaining mixture is dissolved in chloroform, washed three times with a single distilled water, and washed with chloroform Extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com