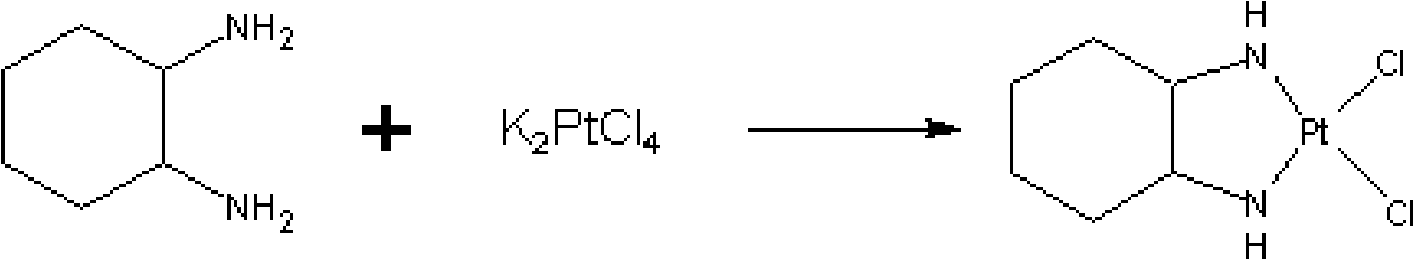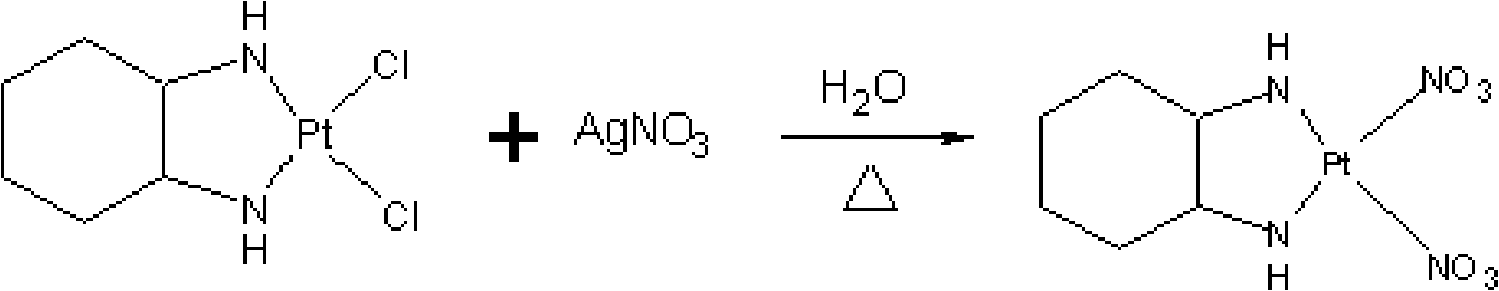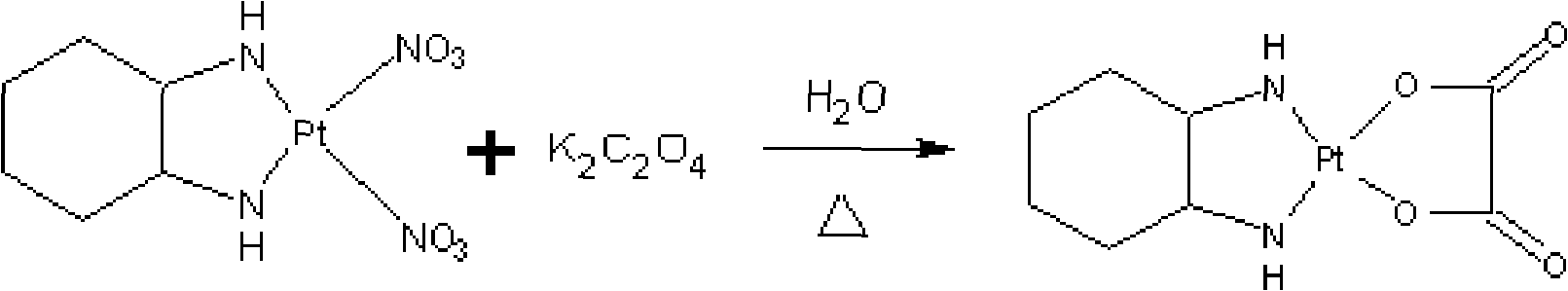Oxaliplatin medicament composition, preparation method thereof and method for synthesizing oxaliplatin as raw medicinal material
A technology of oxaliplatin and composition, which is applied in the field of synthesis of raw material oxaliplatin, can solve the problems of reducing product yield, operator injury, poor molding and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] [Embodiment 1] intermediate (I)---cis-dichloro (trans-(-)-1,2-cyclohexanediamine) synthesis of platinum
[0148] At room temperature, put 10g (4.07mmol) of potassium chloroplatinite into a 150ml three-necked flask, add 100ml of distilled water, and add 2.887g (25.28mmol) of trans-cyclohexane distilled in 15ml of distilled water under stirring. The amine was added dropwise in about 10 minutes, then stirred and reacted at room temperature for 8 hours, and the reaction liquid was detected by thin-layer chromatography to the end point, the reaction solution was filtered, washed three times with 30ml of purified water, once with 20ml of ethanol, once with 20ml of ether, 65 After drying at °C for 4 hours, 8.42 g of a yellow solid product was obtained, with a yield of 91.9%.
Embodiment 2
[0149] [Embodiment 2] Intermediate (II) - the synthesis of cis-dinitro (trans-(-)-1,2-cyclohexanediamine) platinum
[0150] At room temperature, put 8g (21.16mmol) of intermediate I and 6.828g (40.19mmol) of silver nitrate into a 2000ml three-necked flask, add 1200ml of water, blow nitrogen, stir the reaction under dark conditions, and heat up to 50°C. Under reaction for 12 hours, spot plate monitoring, then add 304mg (1.83mmol) of potassium iodide (dissolved in 8ml distilled water) to the there-necked flask, add nitrogen, continue to stir for 1 hour, filter with water membrane, then wash the filter cake with a little purified water, A colorless transparent solution of Intermediate II was obtained.
Embodiment 3
[0151] [Example 3] Synthesis of Oxaliplatin
[0152] Add the intermediate II solution into a 2000ml three-neck flask, protect it with nitrogen, stir it under dark conditions, raise the temperature in a water bath to 50°C, then slowly add 7.80g (42.31mmol) potassium oxalate (dissolved in 60ml distilled water) for about 5min, drop After the addition is complete, add nitrogen, continue the reaction for 3-4 hours, and monitor by pointing the plate. After the reaction is completed, cool the reaction solution to room temperature with water, and filter it with a water membrane to obtain the reaction solution of oxaliplatin. Concentrate under reduced pressure at 65°C to a large amount of solid Precipitate, stop concentrating, cool down with water, filter, and wash the filter cake three times with 40ml×3 purified water, three times with 30ml×3 absolute ethanol, and finally wash with 20ml ether, and dry at 55°C for 4 hours. 6.4 g of white oxaliplatin crystals were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com