Furazolidone metabolite hapten and antigen as well as preparation method and application thereof
A furazolidone and metabolite technology, which is applied in the field of furazolidone metabolite hapten, can solve the problems of expensive instruments, high equipment requirements, and high operator requirements, and achieves great economic value and social significance, high purity and yield, and simple detection. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
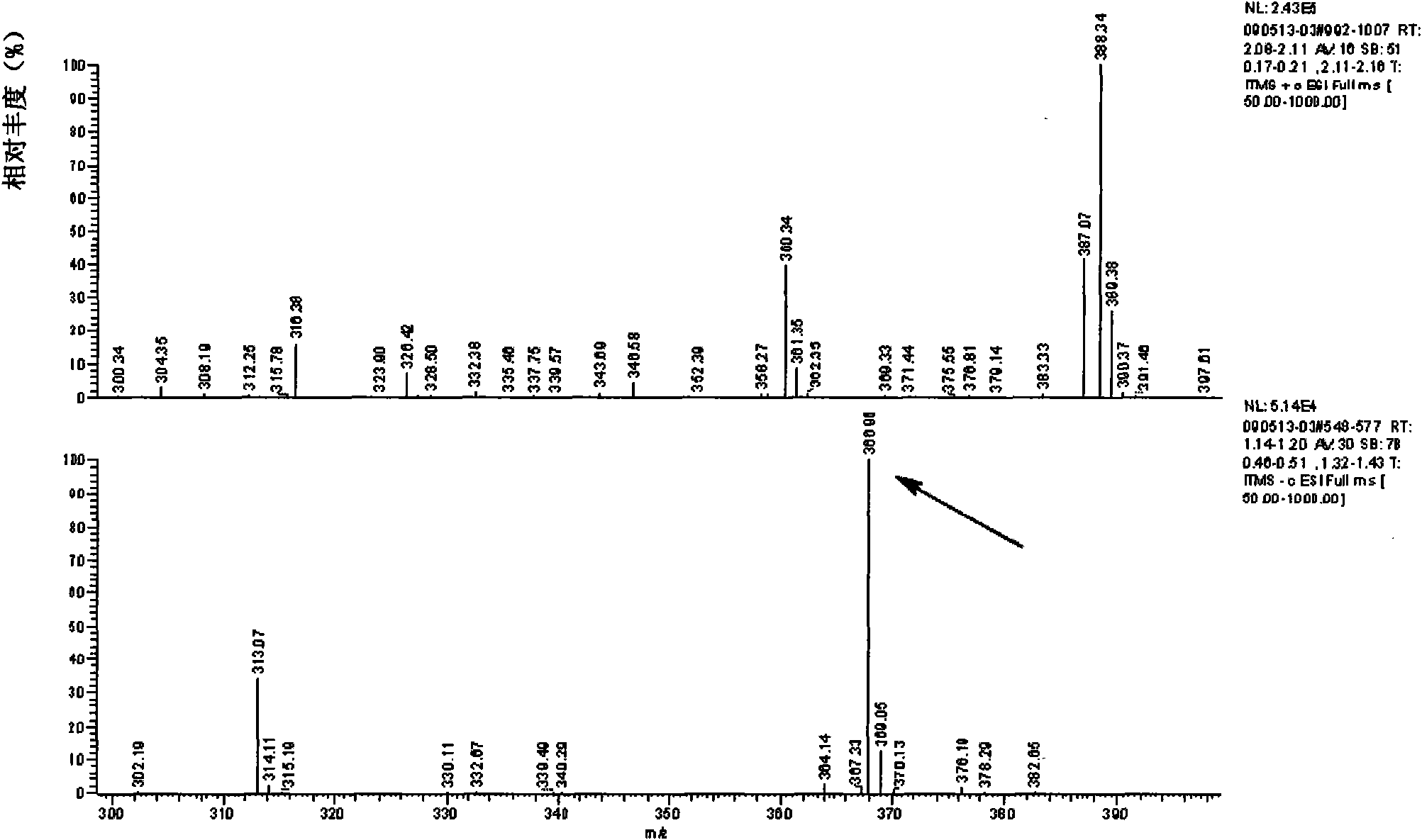
[0037] Example 1, Preparation and identification of furazolidone metabolite hapten
[0038] 1. Preparation of furazolidone metabolite hapten
[0039] 3-amino-2-oxazolidinone: 5-hydroxy-2-nitro-benzaldehyde: succinic anhydride = 1:3.8:8.3 (molar ratio).
[0040] 1. Preparation of solution A and solution B
[0041] Solution A: 25 mg of 3-amino-2-oxazolidinone was dissolved in 1 mL of distilled water, and set aside.
[0042] Solution B: 155mg of 5-hydroxy-2-nitro-benzaldehyde, dissolved in 4mL of ethanol, set aside.
[0043] 2. At room temperature (20-30°C), gradually add solution A to solution B with continuous stirring, and react for 7 hours to obtain solution C.
[0044] 3. The solution C was centrifuged at 5000 rpm for 10 minutes, the precipitate was taken out, washed twice with a small amount of absolute ethanol, and dried in a drying oven at 60°C to obtain a yellow powder.
[0045] 4. Transfer the product of step 3 to a 20 mL round bottom flask, add 200 mg of succinic a...
Embodiment 2
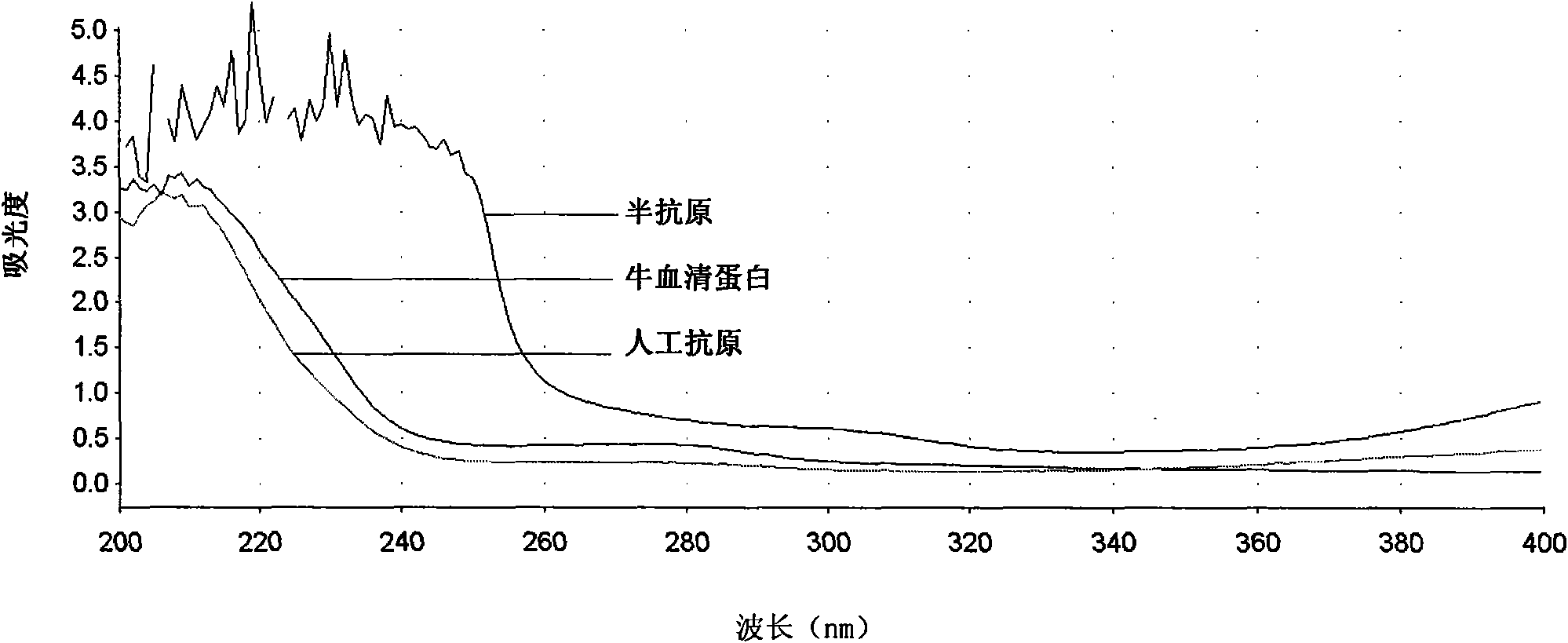
[0052] Example 2, Preparation and Identification of Furazolidone Metabolite Artificial Antigen
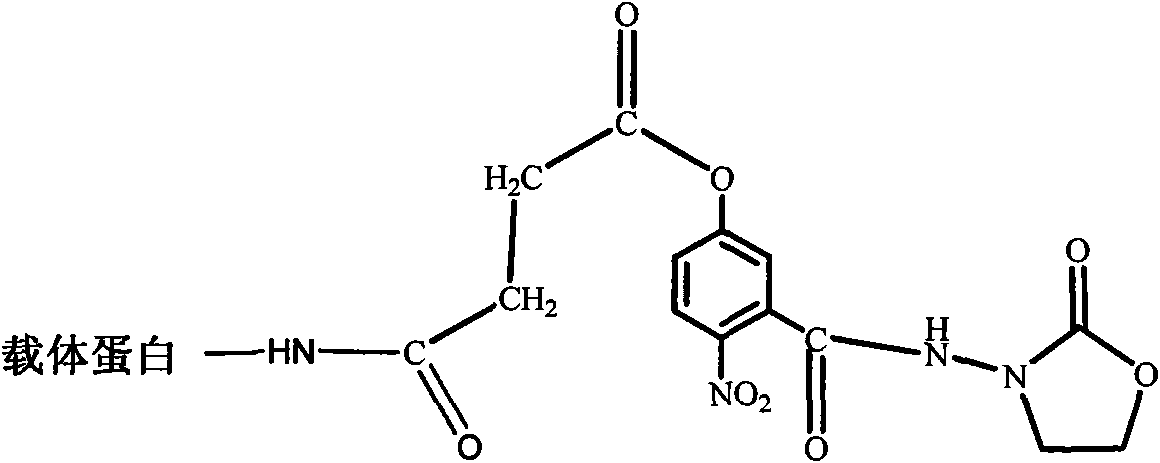
[0053] 1. Preparation of Furazolidone Metabolite Artificial Antigen
[0054]1. Dissolve the furazolidone metabolite hapten (compound shown in formula (I)) prepared in Example 1 in N, N-dimethylformamide (DMF), then add N, N'-dicyclohexylcarbodisulfide Imine (DCC) and N-hydroxysuccinimide (NHS), stirred at room temperature (20-30°C) for 9 hours, overnight at 4°C, centrifuged to separate the supernatant; furazolidone metabolite hapten: DCC: NHS: DMF =1:1:1:4 (molar ratio).
[0055] 2. Add the supernatant to the phosphate buffer (0.01 mol / L, pH 7.4) in which the carrier protein (BSA) is dissolved, and continue to stir for 5 hours to obtain the furazolidone metabolite artificial antigen.
[0056] 3. Put the solution in step 2 into a dialysis bag, dialyze with phosphate buffer (0.01 mol / L, pH 7.4) at 4°C for 72 hours, and change the water 10 times.
[0057] 4. Pass the dialysate thro...
Embodiment 3
[0060] Embodiment 3, preparation and specific identification of antiserum
[0061] 1. Preparation of antiserum from immunized animals
[0062] Five New Zealand white rabbits were used as immunized animals, and the artificial antigen of furazolidone metabolite (prepared in Example 2) was used as the immunogen, and the immunizing dose for each immunization was 1 mg / kg. The immunization method is as follows: at the first immunization, fully emulsify the immunogen with an equal volume of Freund's complete adjuvant, inject it subcutaneously at multiple points on the back of the neck, and take it at intervals of 3-4 weeks. Mix the immunogen with Freund's incomplete adjuvant to make an emulsifier Booster immunization once, a total of 7 times of immunization, the last time without adjuvant.
[0063] From the second booster immunization, blood was collected from the rabbit’s ear vein on the 8th day after each immunization. After the blood was collected, it was placed in a refrigerator...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com