Method for preparing lithium vanadium phosphate as lithium ion battery anode material
A technology for lithium-ion batteries and positive electrode materials, applied in electrode manufacturing, battery electrodes, circuits, etc., can solve the problems of low purity of synthetic products, long reaction time, cumbersome operation, etc., and achieve high machinability and high specific capacity of the electrode sheet , the effect of uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
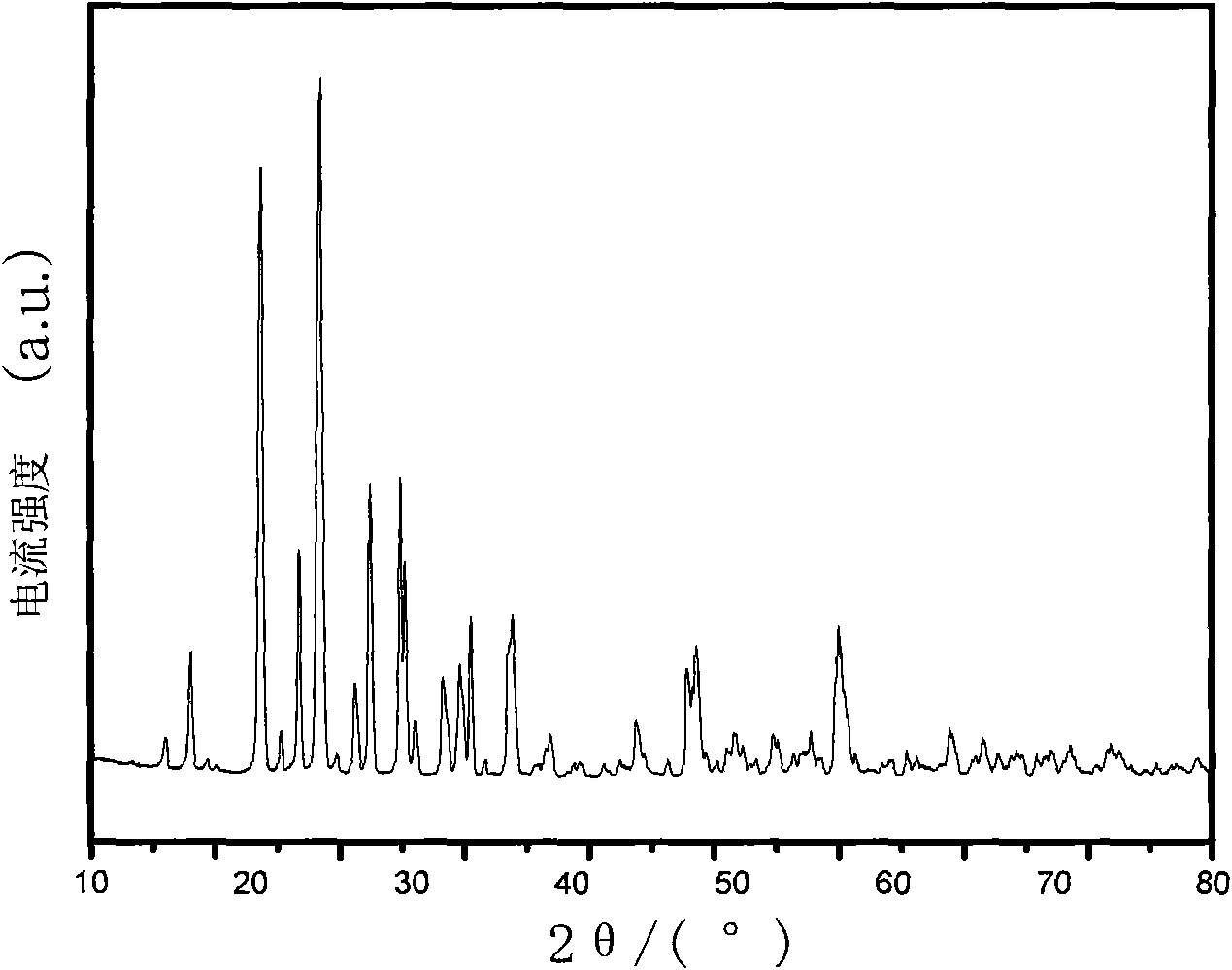
[0033] Will 9.094g V 2 o 5 Dissolve in 18.915 g of oxalic acid, stir and dissolve for 0.5 hour to obtain solution (1). 5.542gLi 2 CO 3 and 17.254 g NH 4 h 2 PO 4 Dissolve in a certain amount of distilled water, stir and dissolve for 0.5 hour to obtain solution (2). The solution (2) was slowly added to the solution (1), and the resulting reaction mixture was stirred and heated and stirred at 80° C. for 4 hours to form a gel. The resulting gel was heated at 350°C, N 2 The heat treatment was carried out for 4 hours under the atmosphere. The resulting material is then pressed into a cylindrical shape under a pressure of 5MPa, and put into a microwave tube reactor of 2KW. 2 Under the atmosphere, the temperature was raised from room temperature to 750° C., and the temperature was kept for 5 minutes. The microwave tubular reaction furnace has an infrared temperature measurement function, which can monitor and automatically adjust the sintering temperature. Then it is natur...
Embodiment 2
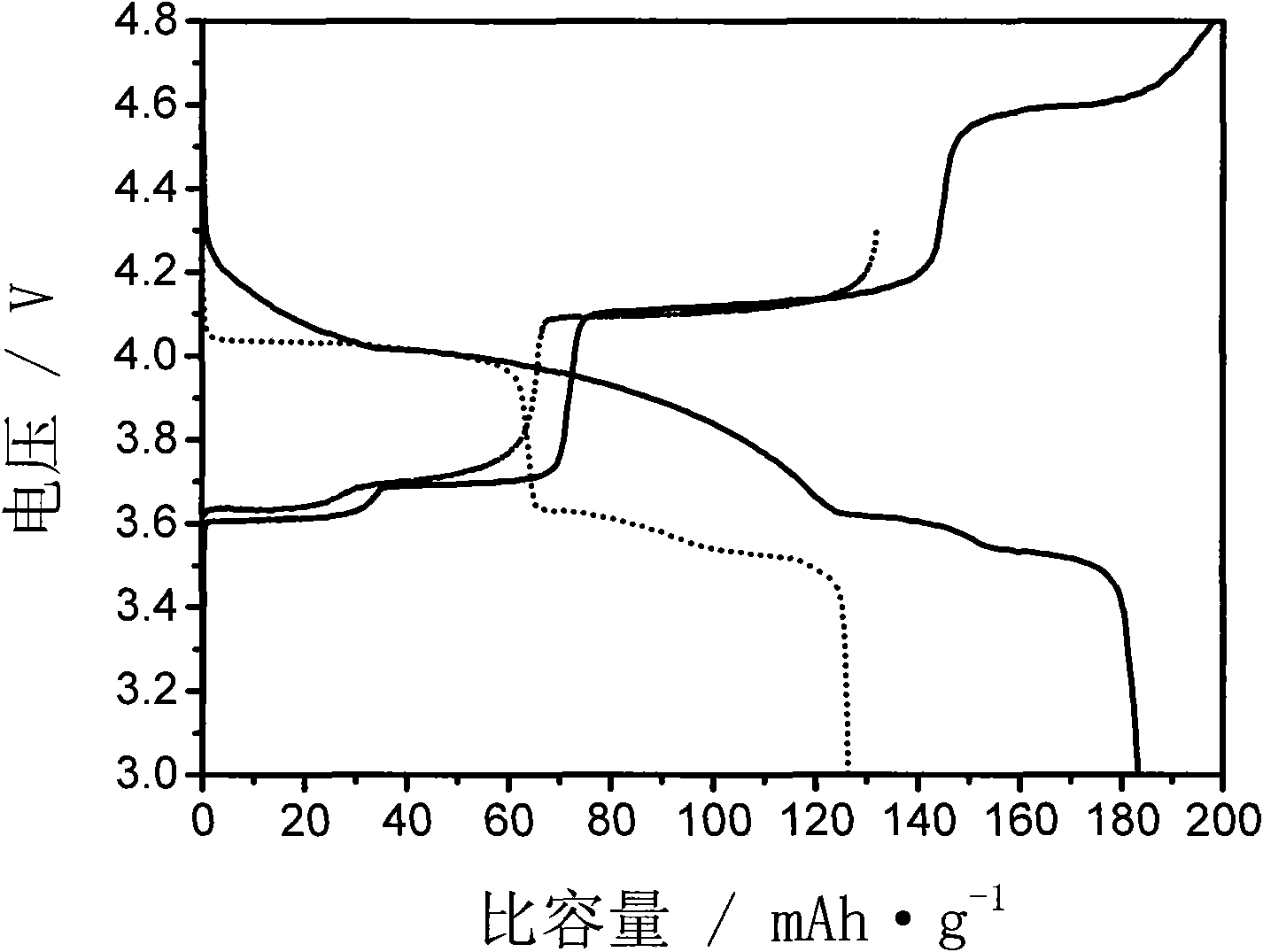
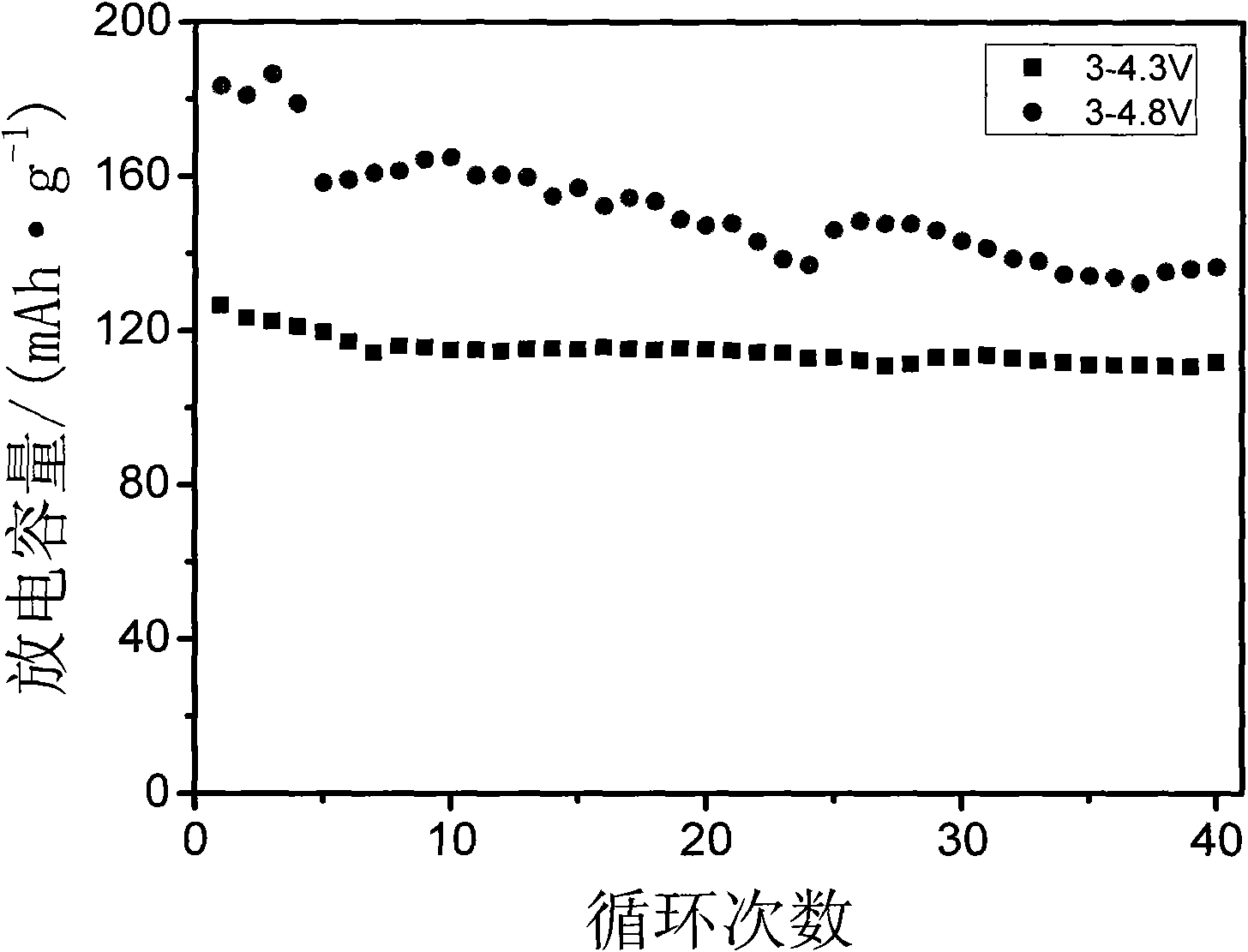
[0037] Change the microwave sintering time to 10 minutes, and other conditions are the same as in Example 1. The resulting product was analyzed by XRD, showing that they were all Li 3 V 2 (PO 4 ) 3 , without any impurity. The resulting product was assembled into a button battery, which was charged and discharged at a rate of 0.1C. The charge and discharge voltages were 3.0-4.3V and 3.0-4.8V. The first discharge capacity was 113.5 and 151.2mAh / g respectively, and the maximum discharge capacity was 177.5mAh / g. g.
Embodiment 3
[0039] Change the microwave sintering time to 20 minutes, and other conditions are the same as in Example 1. The resulting product was analyzed by XRD, showing that they were all Li 3 V 2 (PO 4 ) 3 , without any impurity. The resulting product was assembled into a button battery, charged and discharged at a rate of 0.1C, the charge and discharge voltages were 3.0-4.3V, 3.0-4.8V, and the first discharge capacities were 111.8 and 144.4mAh / g, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com