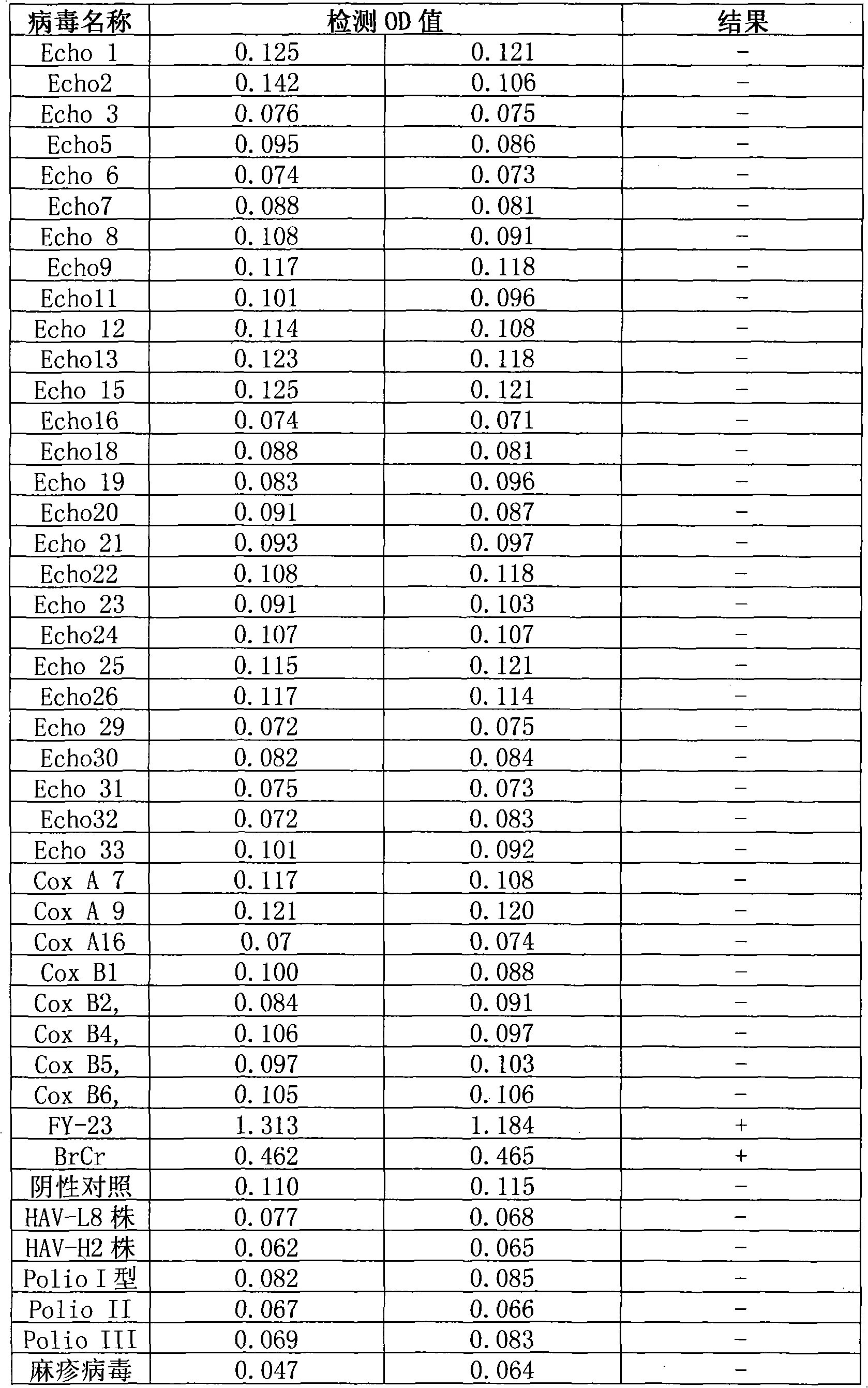
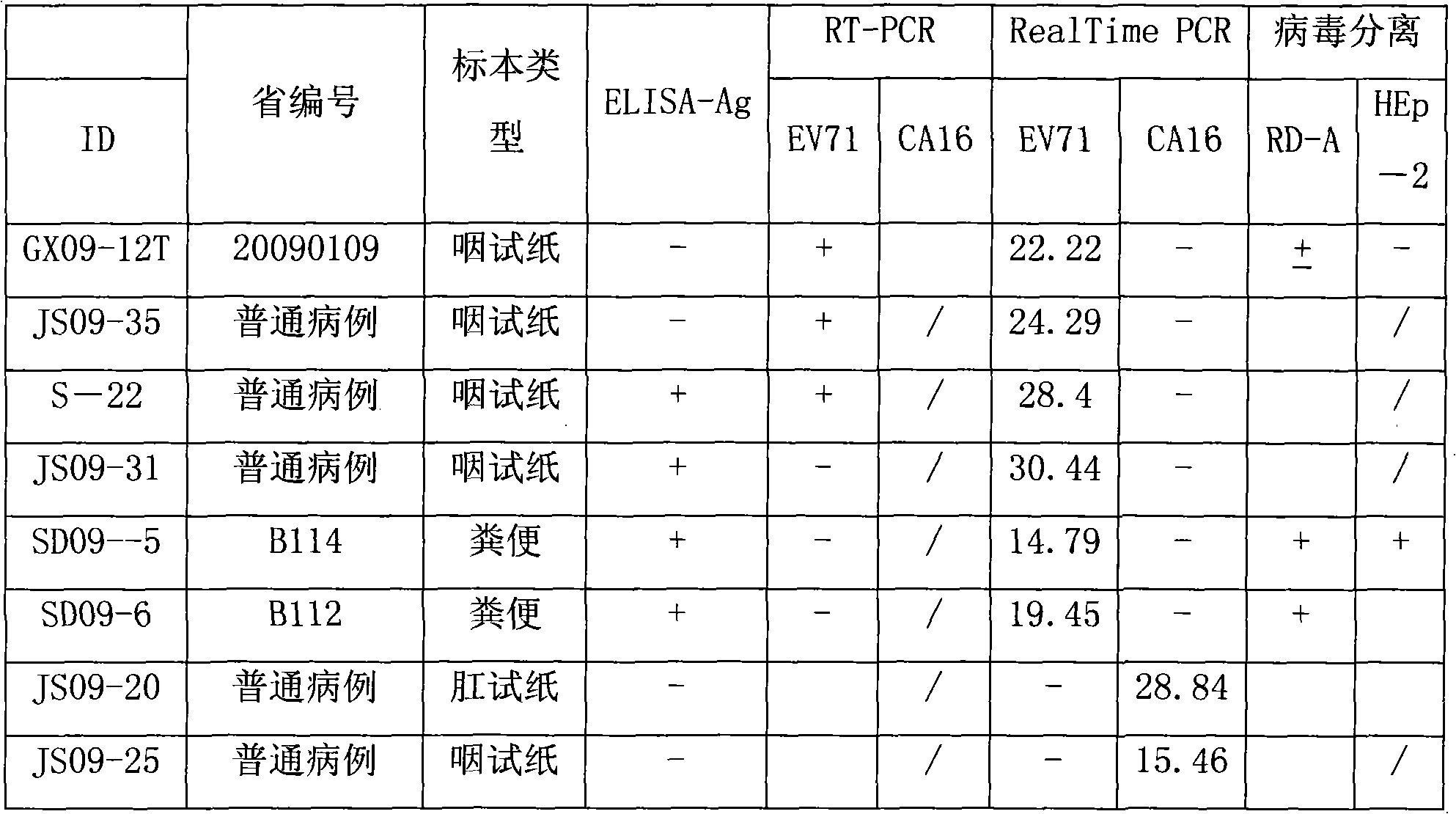
Detection method of EV71 virus antigen
A technology of EV71 and virus antigen, which is applied in the field of biology, can solve the problems of delaying the best treatment time and difficulty in making a clear diagnosis of severe cases, so as to facilitate early diagnosis and control of the epidemic, shorten the detection time, and reduce the number of steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] One-step, separation and purification of EV71 virus antibody
[0051] 1) Take a commercially available protein A / G affinity chromatography column, place it at room temperature to equilibrate for 30 minutes, and equilibrate the column with an equilibration solution 5 times the volume of the column;
[0052] 2) Mix the commercially available anti-EV71 serum with the conventional balance solution at a volume ratio of 1:1 and load the sample, and use 10 times the column volume of the balance solution to elute the impurity protein, leaving the target protein;
[0053] 3) The target protein was eluted from the affinity column with a conventional eluent of 10 times the column volume, the elution peak was collected, and dialyzed overnight with conventional phosphate buffered saline (PBS) to obtain the EV71 virus antibody;
[0054] 4) Use the lowry method to measure the protein content, and store it below -20°C after labeling;
[0055] Two steps, preparation of biotin-labeled E...
Embodiment 2
[0101] One-step, separation and purification of EV71 virus antibody
[0102] With embodiment 1;
[0103] Two steps, preparation of biotin-labeled EV71 antibody
[0104] 1) According to the ratio of biotin: EV71 virus antibody = 1: 20 molar ratio, commercially available biotin was mixed with the one-step EV71 virus antibody, and placed at 5° C. for 2 hours;
[0105] 2) Dialyze the mixture of the above step 1) with phosphate buffered solution (PBS) at 2°C overnight, add an equal volume of glycerol and mix well to obtain biotin-labeled EV71 antibody, and store it below -20°C for future use;
[0106] 3) Dilute the biotin-labeled EV71 antibody in step 2) according to the dilution ratio of 1:100, 1:200, 1:400...1:5000, and determine their optimum by conventional checkerboard test. Using the dilution, the dilution of the biotin-labeled EV71 antibody is 1:500-3000;
[0107] Three steps, the EV71 virus antigen to be tested is obtained through the following methods:
[0108] 1) Coll...
Embodiment 3
[0127] One-step, separation and purification of EV71 virus antibody
[0128] With embodiment 1;
[0129] Two steps, preparation of biotin-labeled EV71 antibody
[0130] 1) According to the ratio of biotin: EV71 virus antibody = 1:5 molar ratio, commercially available biotin was mixed with the one-step EV71 virus antibody, and placed at 8° C. for 1 hour;
[0131] 2) Dialyze the mixture of the above step 1) with phosphate buffered solution PBS at 8°C overnight, add an equal volume of glycerol and mix well to obtain biotin-labeled EV71 antibody, and store it below -20°C for future use;
[0132] 3) Dilute the biotin-labeled EV71 antibody in step 2) according to the dilution ratio of 1:100, 1:200, 1:400...1:5000, and determine their optimum by conventional checkerboard test. Using the dilution, the dilution of the biotin-labeled EV71 antibody is 1:500-3000;
[0133] Three steps, the EV71 virus antigen to be tested is obtained through the following methods:
[0134] 1) Collect t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com