Microgel based on cyclodextrins and preparation method thereof
A technology of cyclodextrin and microgel, which is applied in the direction of medical preparations of non-active ingredients, pharmaceutical formulas, non-effective ingredients of polymer compounds, etc., can solve the problem of not obvious responsiveness to external environmental stimuli and lack of responsiveness to external conditions. , not easy to degrade and other problems, to achieve the effect of controllable molecular weight, narrow molecular weight distribution and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0072] In conjunction with Examples 1 to 3, describe the method for synthesizing polycationic electrolytes based on cyclodextrin
Embodiment 1
[0073] Example 1: β-CD-g-DMAEMA 30 preparation method
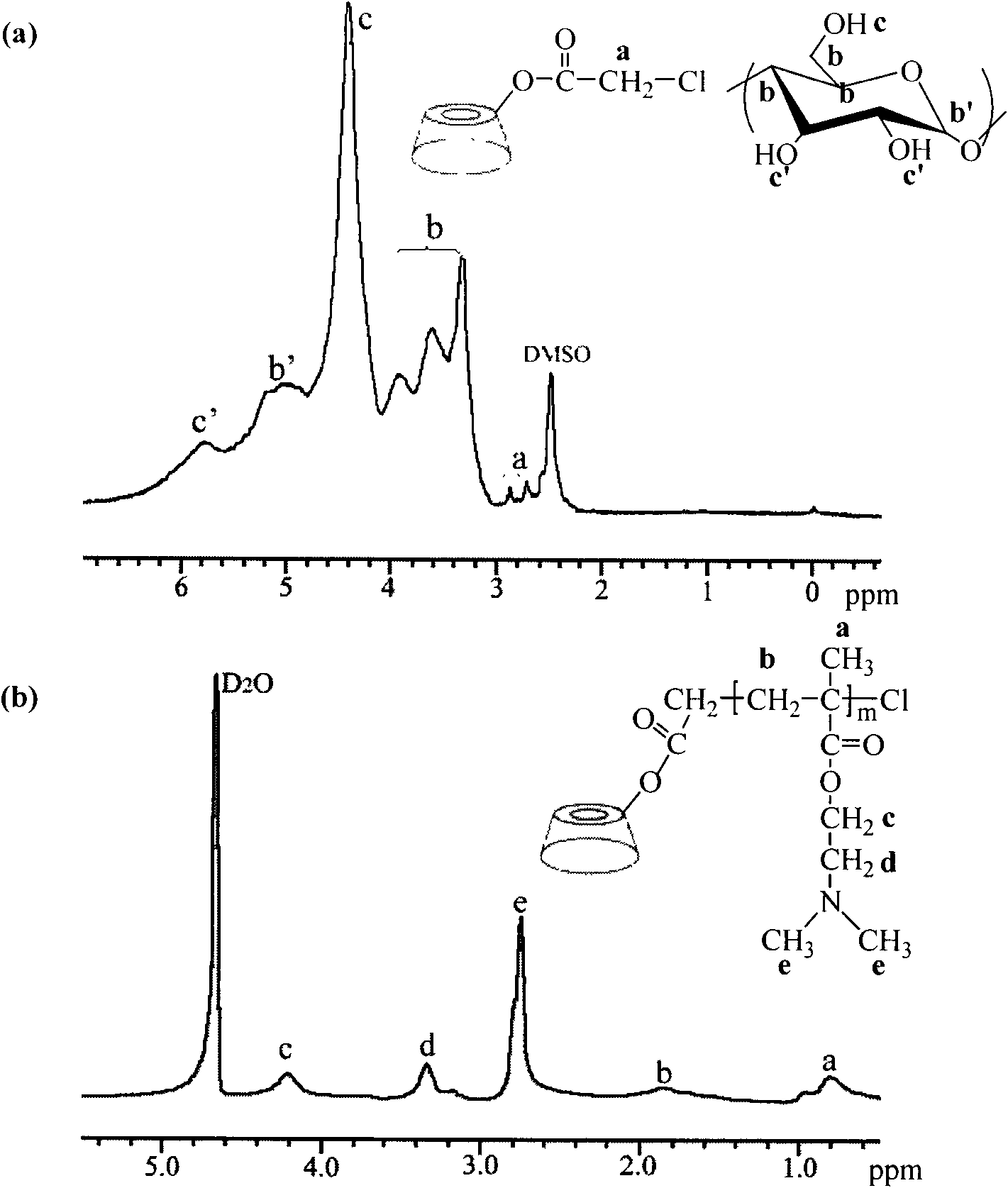
[0074] (1) Preparation of β-CD: Weigh a certain amount of β-cyclodextrin into an ampoule, put it into a vacuum drying oven, set the temperature at 120°C, and dry overnight. Cool immediately in a desiccator after removal.
[0075] (2) ATRP initiator chloroacetylated cyclodextrin (β-CD-Cl 21 ) preparation: put β-CD (4.5mmol, 5g) into a beaker in advance, add 30mL of N,N-dimethylformamide (DMF), and transfer it to a 100mL beaker after β-CD is completely dissolved In a three-necked round-bottomed flask equipped with a reflux condenser, dropping funnel and nitrogen conduit, pass inert gas N 2 After protection, chloroacetyl chloride (0.22 mol, 25 g) dissolved in 10 mL of DMF was slowly added dropwise to the β-CD solution under magnetic stirring. The dropwise reaction was carried out at room temperature. After the dropwise addition, the oil bath was heated to 80° C., and the system was reacted at this temperature for 24 hou...
Embodiment 2
[0082] Example 2: γ-CD-g-DMAEMA 30 preparation method
[0083] (1) Preparation of γ-cyclodextrin and ATRP initiator based on γ-cyclodextrin (γ-CD-Cl 21 ) preparation and purification: with embodiment one.
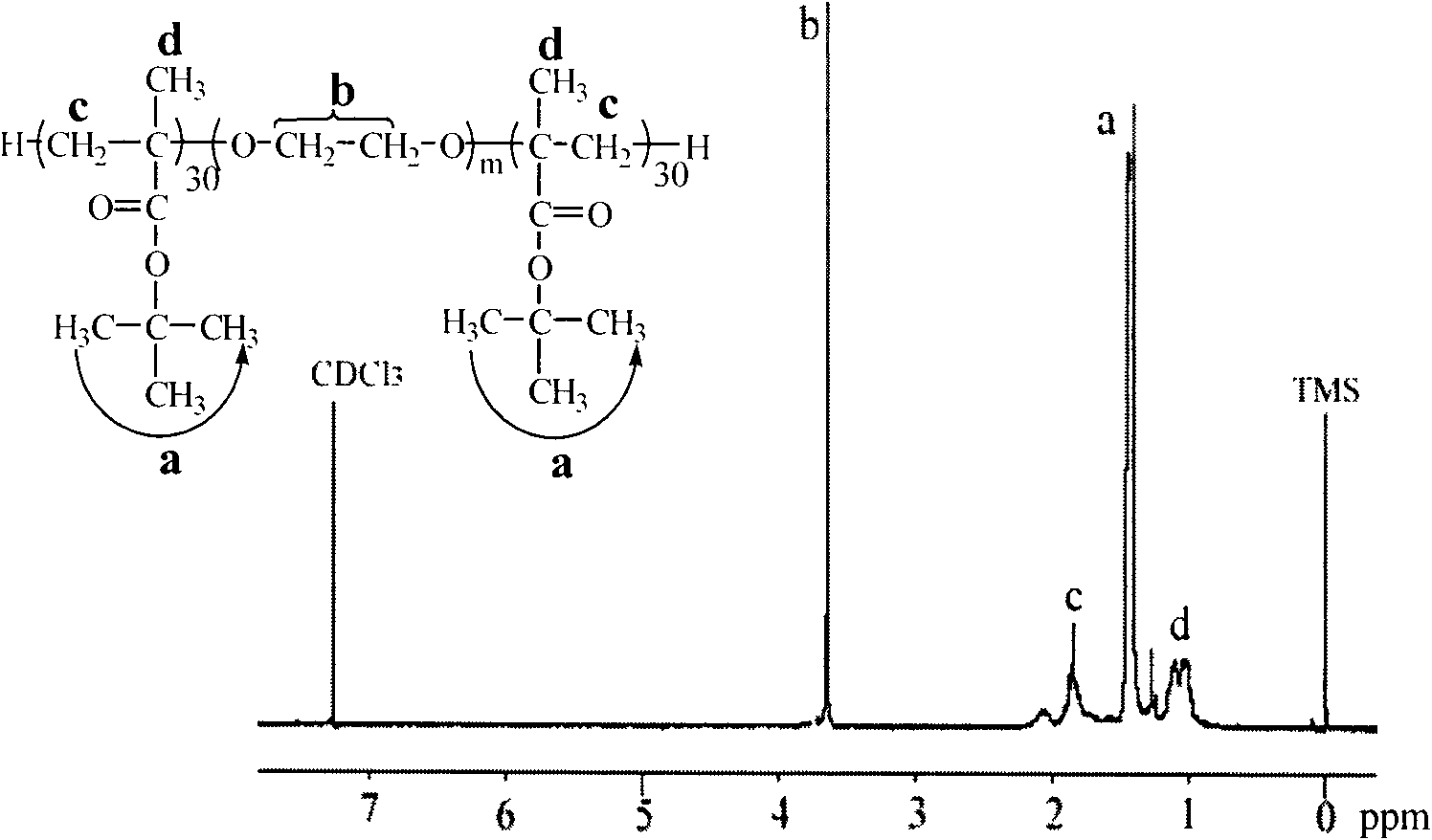
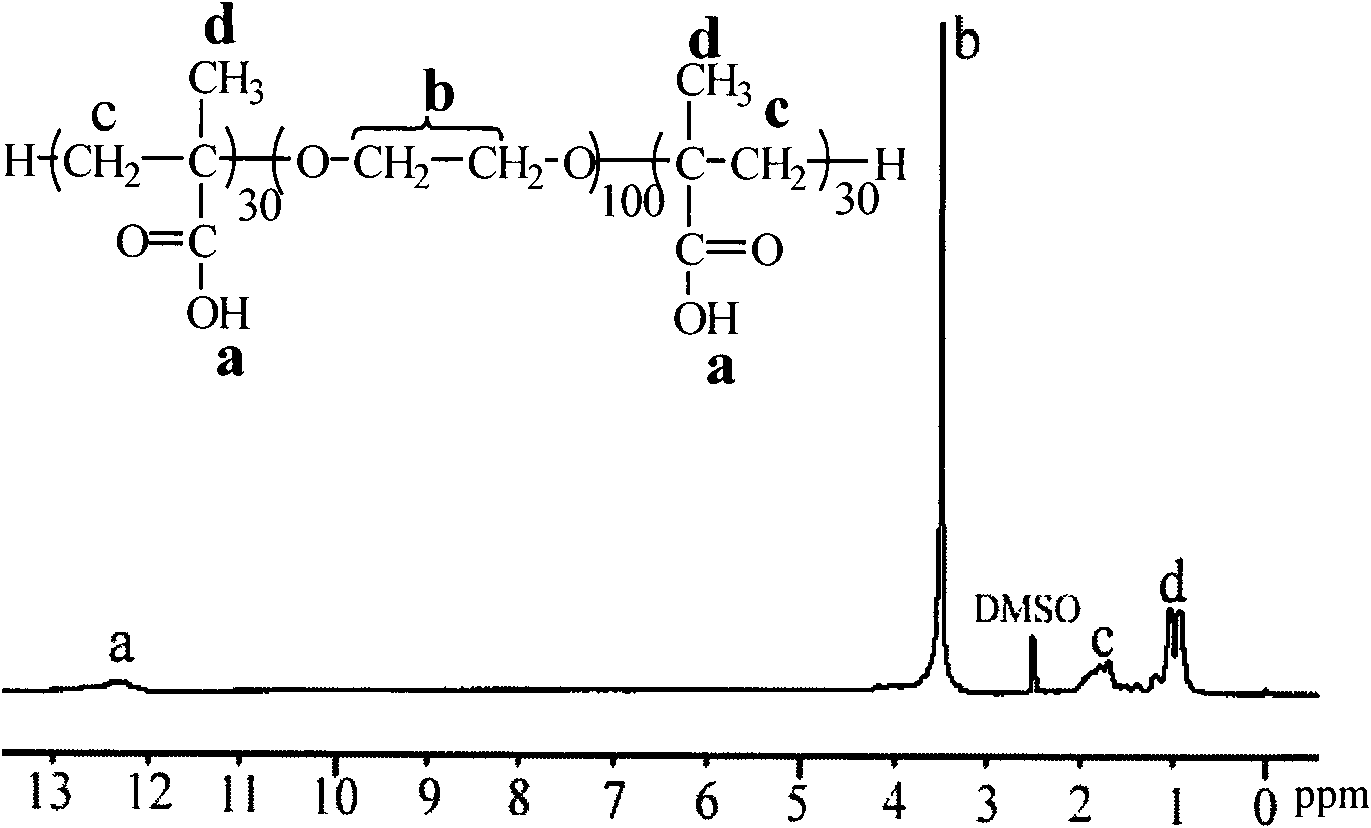
[0084] (2)γ-CD-g-DMAEMA 30 Preparation: Put the vial in an oven at 120°C to dry in advance, take it out and plug it with a ground glass stopper, and cool it with a pump until it reaches room temperature. The dry initiator γ-CD-Cl 21 (0.046mmol) and ligand 2,2'-bipyridine (2.50mmol) were added in the branch bottle, stirred evenly, and then the hydrophilic monomer methacrylate-2-(dimethylamino)ethyl (DMAEMA) ( 37.50 mmol) was added into the mixture containing the initiator and the ligand with a syringe, stirred and dissolved, vacuumized, and then high-purity argon was introduced, and repeated three times. Add 8 mL of deionized water with a syringe, vacuumize, then flush with argon, stir and dissolve until the mixture solution is transparent. Finally, purified cuprous br...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com