Human genetic engineering antibody TRD 109 as well as preparation method and application thereof
A genetically engineered antibody, antibody technology, applied in the direction of antibodies, anti-inflammatory agents, recombinant DNA technology, etc., can solve the problems of heavy economic burden, high treatment costs, low immunogenicity, etc. Simple structure and the effect of relieving symptoms of allergic rhinitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Construction of ribosome-displayed single-chain antibody (VH / K) gene library
[0057] 1-1 Extraction of total cellular RNA and synthesis of cDNA
[0058] Extract 40ml of peripheral blood from patients clinically diagnosed as rheumatoid arthritis, add 5U heparin to each milliliter of blood; ) in a centrifuge tube, put it in a low-speed desktop centrifuge, 2500r / min, and centrifuge for 20min; gently absorb the lymphocyte layer with a capillary pipette, and place it in another 15ml centrifuge tube; dilute it with normal saline, 1000r / min, Centrifuge for 10 min, carefully remove the supernatant, and repeat this process twice; resuspend the pellet according to the ratio of adding 1 ml of normal saline to every 10 ml of blood, and distribute it in EP tubes; then use the total RNA isolation kit (Promega Company) to extract the total RNA of the cells .
[0059] Synthesis of cDNA (RNA reverse transcription kit purchased from TaKaRa Company) using total cell RNA as template, Hu...
Embodiment 2
[0084] In vitro transcription and translation
[0085] The in vitro transcription and translation were carried out by expresswayTM Plus ExpressionSystem of Invitrogen Company according to the operation manual. 20μL of IVPS Plus E.coli Extract, 20μL of 2.5×IVPS Plus E.coli Reaction Buffer, 1μL of 75mmol / L methionine, 1μL of 200μmol / L Anti-ssrA oligonucleotide, 1μL of PCR product, and 6μL of DNase-free water Add it into a 2.0mL EP tube; keep the reaction solution at 37°C for 30min, and use 4 times the volume of ice-precooled WBTH (50mmol / L Tris-acetate Ph 7.5, 150mmol / L NaCl, 50mmol / L magnesium acetate, 0.1% Tween-20, 2.5g / Lheparin, Sigma) After terminating the reaction, add pre-cooled 1 / 5 volume of WBT containing 10% (w / v) BSA to the supernatant; the reaction mixture was centrifuged at 1400g for 5min at 4°C Afterwards, transfer the supernatant to a new ice-cooled EP tube.
Embodiment 3
[0087] Affinity screening of ribosomal complexes
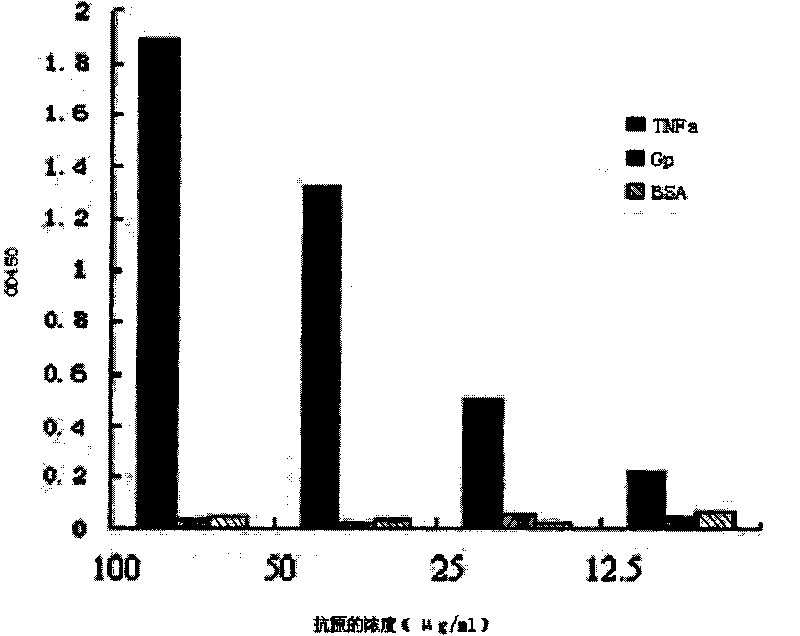
[0088] In order to screen the specific VH / K antibody gene against TNF-α, the 96-well plate was coated with recombinant TNF-α purified protein (PEPROTECH, USA), 40 μg / well, BSA-S.cerevisiae RNA-PBS blocking; the translation mixture Transfer to the coated wells, shake the microplate in a cold room for 1 hour, pour off the translation reaction solution, wash the microplate with WBT for 5 times; Shake for 5 minutes to elute the mRNA; the eluted mRNA was purified with RNeasy kit; the purified mRNA was dissolved in RNase-free water and treated with RNase-free DNase to remove the DNA molecules in the reaction solution; the purified mRNA was used as a template, RT-PCR was carried out, and the PCR product was used as the next cycle, namely: transcription-translation-screening, and a total of 3 rounds of screening were performed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com