Medicine composite for treating vaginitis
A composition and vaginitis technology, applied in the field of pharmaceutical preparations, can solve the problems of affecting the curative effect, easy leakage, low curative effect, etc., and achieve the effects of improving drug compliance, prolonging contact time, and prolonging release time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
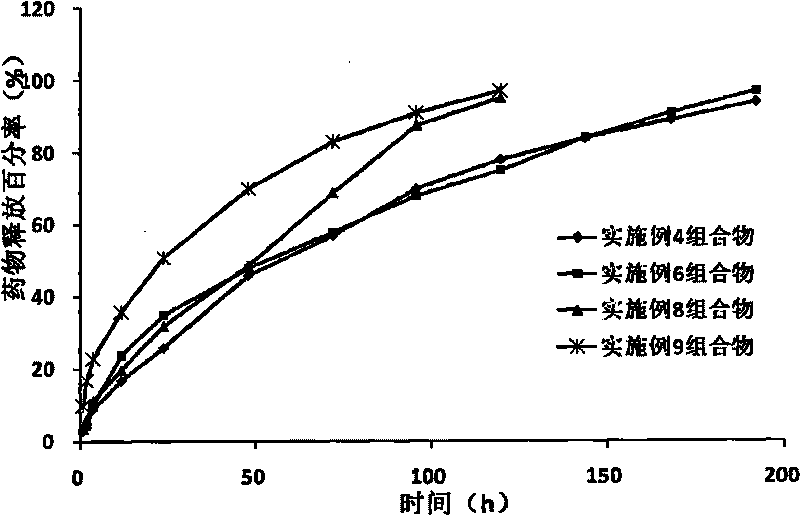
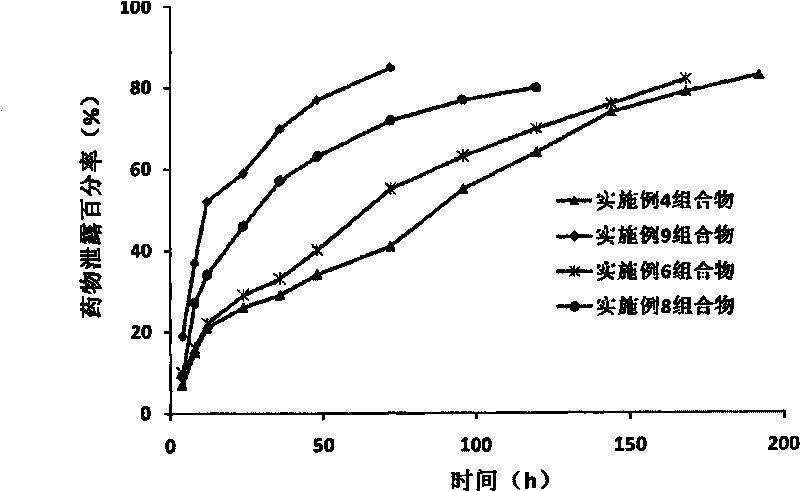
Image
Examples
Embodiment 1
[0073] Prescription composition:
[0074] Butoconazole Nitrate 2%
[0075] Poloxamer F127 17%
[0076] Carbomer 934 0.5%
[0077] Hypromellose K4M 1.5%
[0078] Methylparaben 0.15%
[0079] water balance
[0080] Preparation:
[0081] Take methyl paraben, add water to dissolve and stir evenly, add poloxamer, carbomer and hypromellose while stirring, place it at 4°C, after fully dissolved, add 200-mesh sieve Stir the butoconazole nitrate evenly, pack it into vaginal applicators, 5g each, and get it.
Embodiment 2
[0083] Prescription composition:
[0084] Butoconazole Nitrate 100mg
[0085] Hypromellose K4M 400mg
[0086] Carbomer 947 100mg
[0087] Macrogol 4000 200mg
[0088] Preparation:
[0089] 1. Take butoconazole nitrate and pass through a 200-mesh sieve, hypromellose, polyethylene glycol and carbomer respectively pass through a 80-mesh sieve, mix well, add 80% ethanol solution to make a suitable soft gel, and pass through a 18-mesh sieve Make granules and dry them at 40-50°C to obtain dry granules;
[0090] 2. Pass the dry granules through a 20-mesh sieve for granulation, add talcum powder (according to 1% of the total amount of granules) as a flow aid and lubricant, mix well, and measure the granule content;
[0091] 3. Press into tablets, each tablet contains 100 mg of butoconazole nitrate, ready to pack.
Embodiment 3
[0093] Prescription composition:
[0094] Butoconazole Nitrate 1%
[0095] Hydroxypropyl Beta Cyclodextrin 40%
[0096] Poloxamer F127 17%
[0097] Hypromellose K4M 2%
[0098] Methylparaben 0.5%
[0099] Propylparaben 0.5%
[0100] Edetate Disodium 0.01%
[0101] water balance.
[0102] Preparation:
[0103] Take butoconazole nitrate and hydroxypropyl beta cyclodextrin, add water and continue to stir to dissolve, add edetate disodium, methylparaben and propylparaben, stir to dissolve, add poloxa while stirring Mulberry and hypromellose are placed at 4°C, and after being fully dispersed, they are divided into vaginal applicators, 10g each, and ready to be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com