Water-soluble perylene diimide derivatives containing N-pyridine oxide groups and synthetic method thereof
A technology for oxidizing pyridine groups and pyridyl groups, which is applied in the field of water-soluble perylene imide derivatives and their synthesis, can solve the problems of high cost of target compounds, low fluorescence quantum yield, damage to equipment, etc., and achieve low toxicity, The effect of high fluorescence quantum yield and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
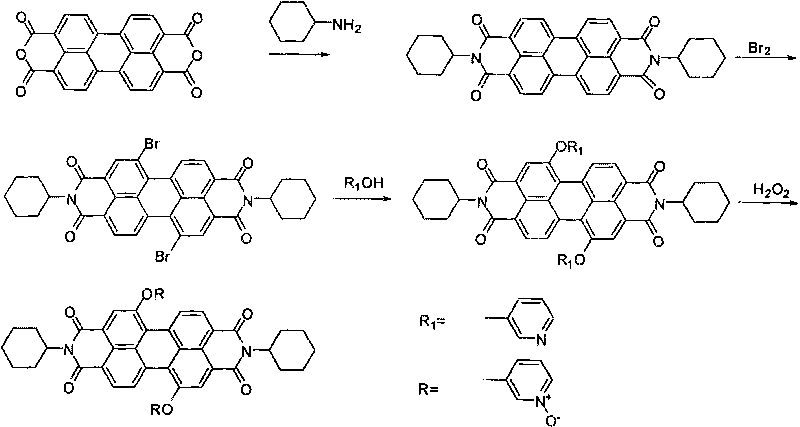
[0030] 1,7-bis(3-pyridyloxy)-N,N'-dicyclohexyl-3,4:9,10-perylene diimide preparation (synthetic route see figure 1 ):
[0031] Add 2.00 grams of 3,4:9,10-perylenetetracarboxylic dianhydride and 30 milliliters of cyclohexylamine to a 100 milliliter reaction flask, heat and reflux under vigorous stirring until the solid is completely dissolved, and evaporate the excess cyclohexylamine under reduced pressure for about 40 hours. Hexylamine was recovered and dried to obtain 2.70 g of the product N,N'-dicyclohexyl-3,4:9,10-perylenetetracarboxylic acid diimide, with a yield of 96%;
[0032] Add 2.00 g of N,N'-dicyclohexyl-3,4:9,10-perylenetetracarboxylic acid diimide and 150 ml of dichloromethane to a 250 ml reaction bottle, then add 30 ml of 10 gram of bromine in dichloromethane solution, heated and refluxed for 4 days under the protection of argon, cooled to room temperature, and the mixed solution of dichloromethane and bromine was evaporated under normal pressure for recovery, s...
Embodiment 2
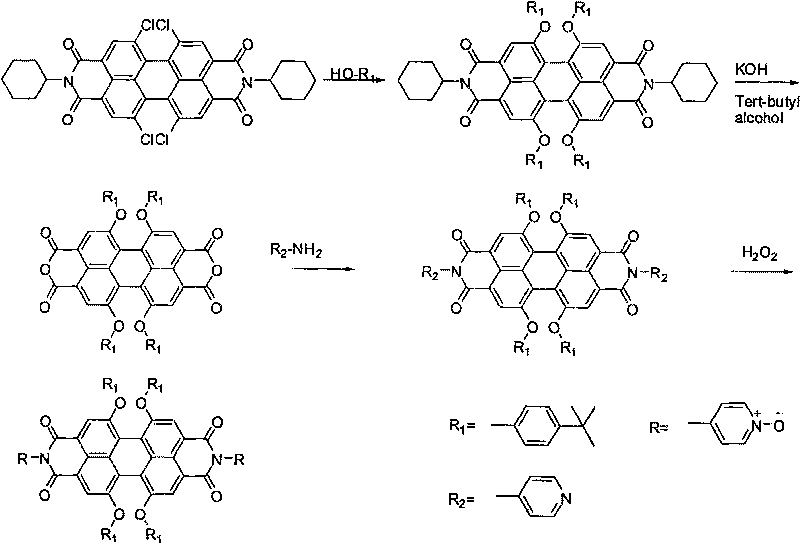
[0039] Preparation of 1,6,7,12-tetra-tert-butylphenoxy-N,N'-bis(4-oxypyridinyl)-3,4:9,10-perylenetetracarboxylic acid diimide (synthetic route see figure 2 ):
[0040] Add 1.00 g of 1,6,7,12-tetrachloro-N, N'-dicyclohexyl-3,4:9,10-tetracarboxylic acid diimide, 1.74 g of tert-butyl Phenol, then add 0.97 grams of potassium carbonate, 20 milliliters of N-methylpyrrolidone (NMP), react at 110 ° C for 24 hours, cool to room temperature, pour the solution into 150 milliliters of 20% HCl solution, let stand overnight, filter with suction, wash with water until Neutral, then washed with a small amount of methanol, dried, and separated by column chromatography, dichloromethane: petroleum ether = 1: 1 as eluent, to obtain 1.07 g of product 1,6,7,12-tetra-tert-butylphenoxy -3,4: 9,10-perylenetetracarboxylic acid diimide, yield 65%;
[0041] Add 0.50 g of 1,6,7,12-tetra-tert-butylphenoxy-N,N'-dicyclohexyl-3,4:9,10-perylenetetracarboxylic diimide to a 50 ml reaction bottle Amine, 30 m...
Embodiment 3
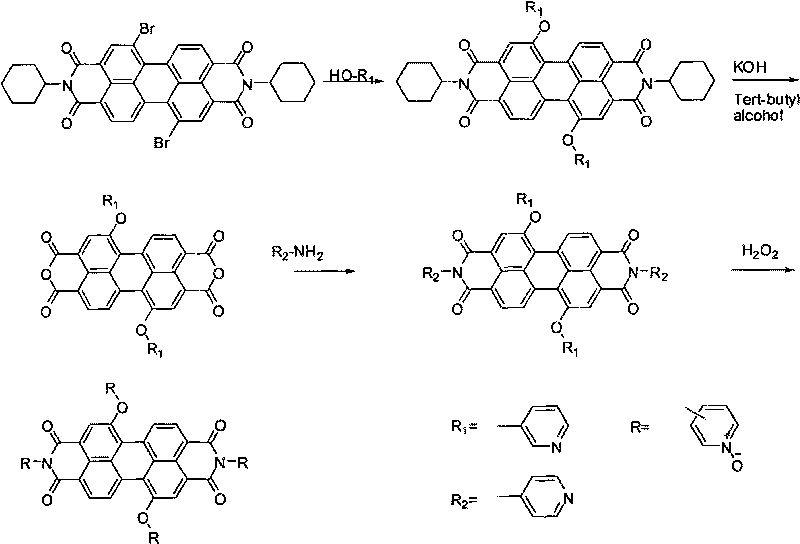
[0047] 1,7-bis(3-pyridyloxy)-N,N'-bis(4-nitrooxypyridyl)-3,4:9,10-perylenetetracarboxylic acid diimide (synthetic route see image 3 ):
[0048] Add 0.70 g of 1,7-dibromo-N, N'-dicyclohexyl-3,4:9,10-tetracarboxylic acid diimide, 0.55 g of m-hydroxypyridine, and then add 0.81 gram of potassium carbonate, 20 milliliters of N-methylpyrrolidone (NMP), reacted for 24 hours at 80° C., cooled to room temperature, poured the solution into 150 milliliters of 20% (wt %) HCl solution, stood still overnight for suction filtration, and washed with water until neutral , washed with a small amount of methanol, dried, and separated by column chromatography, using dichloromethane: acetone = 20:1 as the eluent to obtain 702 mg of product 1,7-bis(3-pyridyloxy)-3,4:9 , 10-perylenetetracarboxylic acid diimide, yield 80%;
[0049] Add 0.60 g of 1,7-bis(3-pyridyloxy)-N,N'-dicyclohexyl-3,4:9,10-perylenetetracarboxylic acid diimide to a 50 ml reaction bottle, 30 ml Isopropanol, then add 1.36 g of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com