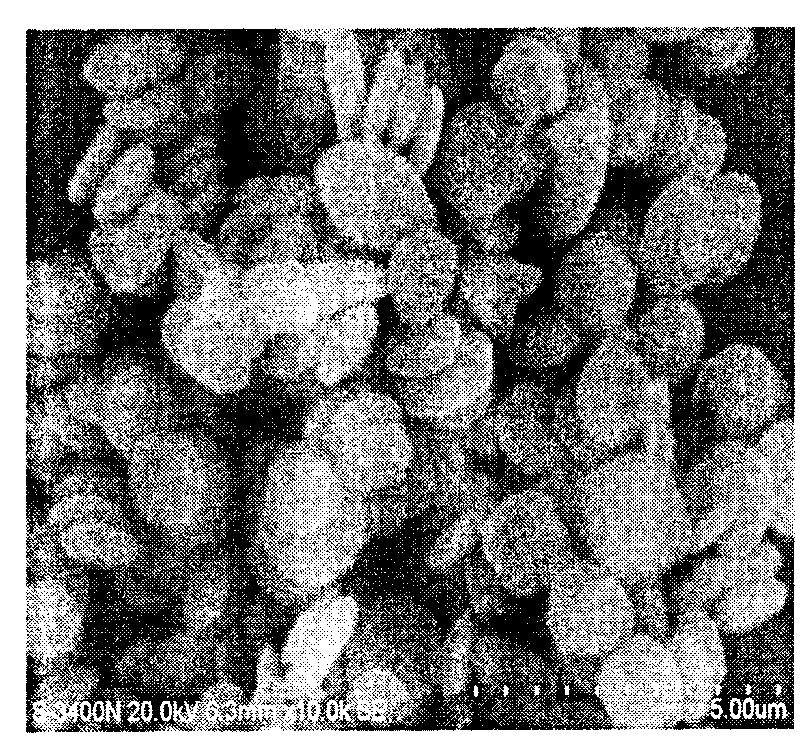Wafer-like ferric phosphate, preparation method and application thereof
A technology of iron phosphate and iron phosphate powder, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of irregular particle shape, uneven size, high impurities, etc., achieve short reaction time and easy process , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 1 liter of deionized water adjusted to pH=1 with nitric acid in advance into the stirred reactor, then add 40.4g (0.1mol) Fe(NO 3 ) 3 9H 2 O, to be Fe(NO 3 ) 3 9H 2 After O is dissolved, add 14.2g (0.1mol) Na 2 HPO 4 , 9g (0.15mol) urea, 3g nonylphenol polyoxyethylene (10) ether, and adjust the pH of the solution to 1-1.5 with nitric acid. Heat the reactor solution to 80°C and react at this temperature for 3 hours to obtain a white suspension, cool, filter, wash the filter cake with deionized water three times, and dry the filter cake in an oven at 100-120°C for 6 hours , and iron phosphate powder can be obtained. The product has good whiteness, disc shape, average particle size 0.3-0.5 microns, tap density ≥ 0.95g / cm 3 .
[0017] The FePO prepared in this embodiment 4 with LiOH·H 2 O, sucrose is mixed with the ratio of 1: 1: 1.15 (ratio of the amount of substance), ball milled 4h, puts into the program temperature control tube furnace in high-purity N 2...
Embodiment 2
[0021] Add 1 liter of deionized water adjusted to pH=1 with nitric acid in advance into the reactor with stirring, then add 202g (0.5mol) Fe(NO 3 )3 9H 2 O, to be Fe(NO 3 ) 3 9H 2 After O is dissolved, add 87g (0.5mol) K 2 HPO 4 , 36g (0.6mol) urea, 3g octylphenol polyoxyethylene (10) ether, adjust the pH=1 of the solution with nitric acid. Heat the reactor solution to 80°C and react at this temperature for 3 hours to obtain a white suspension, cool, filter, wash the filter cake with deionized water three times, and dry the filter cake in an oven at 100-120°C for 5 hours , and iron phosphate powder can be obtained. The product has good whiteness, disc shape, average particle size 0.3-0.5 micron, tap density ≥ 0.95g / cm 3 . The lithium iron phosphate synthesized from this iron phosphate has a discharge capacity of 142mAh / g at 0.5C.
Embodiment 3
[0023] Add 1 liter of deionized water adjusted to pH=1 with nitric acid in advance into the reactor with stirring, then add 404g (1.0mol) Fe(NO 3 ) 3 9H 2 O, to be Fe(NO 3 ) 3 9H 2 After O is dissolved, add 66g (0.5mol) (NH 4 ) 2 HPO 4 , 71g (0.5mol) Na 2 HPO 4 , 72g (1.2mol) urea, 5g cetyltrimethylammonium bromide, adjust the pH of the solution to 1-1.5 with nitric acid, heat the reactor solution to 90°C and react at this temperature for 2 hours to obtain a white suspension The turbid liquid is cooled, filtered, the filter cake is washed three times with deionized water, and the filter cake is baked in an oven at 100-120° C. for 4 hours to obtain iron phosphate powder. The product has good whiteness, disc shape, average particle size 0.3-0.5 microns, tap density ≥ 0.95g / cm 3 . The lithium iron phosphate synthesized from this iron phosphate has a discharge capacity of 142mA·h / g at 0.5C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com