Medicament eluting stent and preparation method thereof
A technology for eluting stents and drugs, applied in the field of stents, can solve problems such as increasing inflammatory reactions and adverse reactions, and achieve the effects of reducing restenosis, reducing late thrombus, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
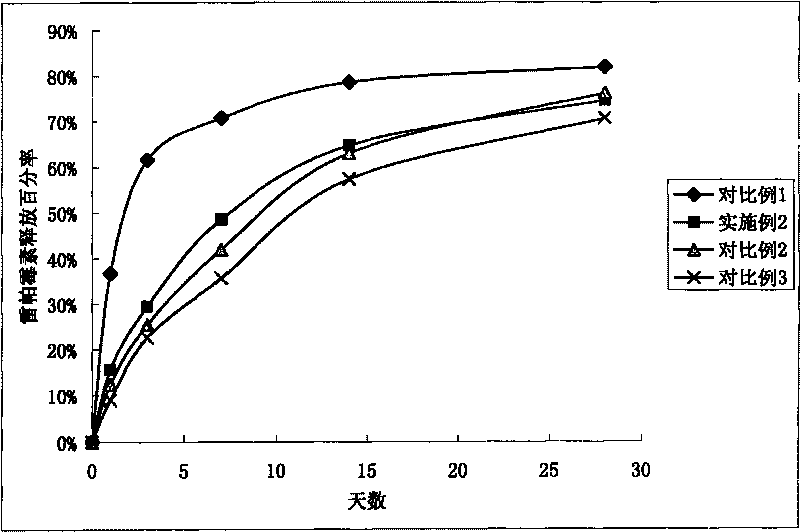
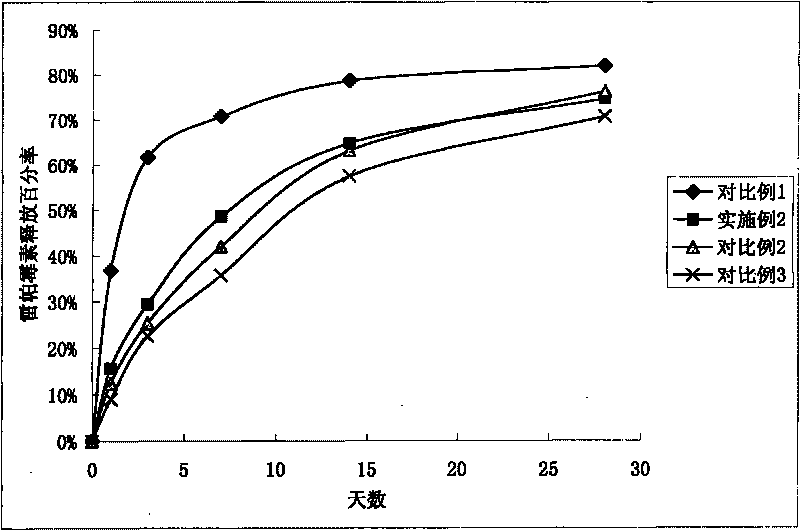
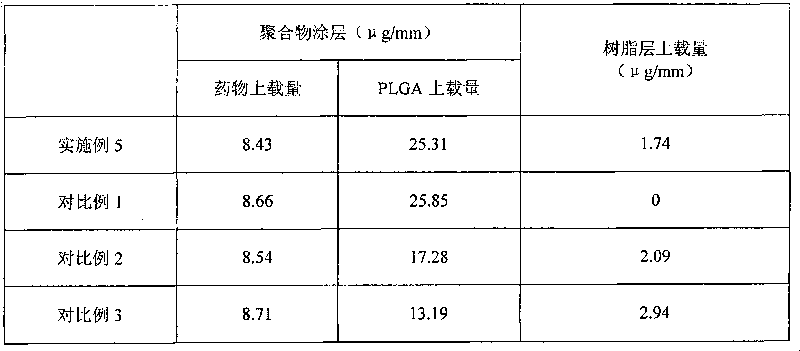
Image
Examples
Embodiment 1
[0068] Lactic acid-glycolic acid copolymer (PLGA, lactic acid / glycolic acid=50 / 50, molecular weight 50000) was dissolved in methylene chloride to make a 0.05wt% solution, sprayed onto the surface of 316 stainless steel stents, after spraying, put it on Vacuum drying at 42° C. for half an hour to form the bottom layer of the stent coating. The bottom layer of the stent coating has a PLGA weight of 0.05 μg / mm per unit length in the axial direction of the stent.
[0069] Lactic acid-glycolic acid copolymer (PLGA, lactic acid / glycolic acid=50 / 50, molecular weight 50000) was dissolved in dichloromethane to make 0.5wt% solution, and drug rapamycin was dissolved therein to make 0.2wt % solution, using a spraying machine to spray, after spraying, it was vacuum dried at 42°C for half an hour to form a scaffold polymer layer. Drug / polymer weight ratio=1:2.5, the weight of PLGA and rapamycin in the stent polymer layer is 31.11 μg / mm per unit length in the axial direction of the stent.
...
Embodiment 2
[0073] Lactic acid-glycolic acid copolymer (PLGA, lactic acid / glycolic acid=75 / 25, molecular weight 80000) was dissolved in acetone to make a 1.0wt% solution, and the drug paclitaxel was dissolved therein to make a 0.05wt% solution, dip coating onto the surface of the nickel-titanium alloy stent, and vacuum-dry it at 42°C for half an hour to form a polymer layer of the stent, with a drug / polymer weight ratio=0.05:1, and the PLGA and drug weights of the stent polymer layer are the The weight per unit length in the axial direction of the stent was 186.67 μg / mm.
[0074] Shellac resin (molecular weight 2000) is dissolved in ethanol to make 0.5wt% solution, and shellac resin is coated on the drug-loaded polymer surface in the same way, and its weight is that the weight per unit length in the axial direction of the stent is 6.67 μg / mm.
[0075] The prepared scaffolds were vacuum-dried at 42°C for 48 hours.
Embodiment 3
[0077] Lactic acid-glycolic acid copolymer (PLGA, lactic acid / glycolic acid=90 / 10, molecular weight 120000) was dissolved in THF to make a 5.0wt% solution, and the filtrate was brushed onto the outer surface and side cracks of the cobalt-chromium alloy stent with a brush On the surface, it was vacuum-dried at 42° C. for half an hour to form the bottom layer of the stent, and the weight of the PLGA on the bottom layer of the stent was 4.78 μg / mm per unit length in the axial direction of the stent.
[0078] Lactic acid-glycolic acid copolymer (PLGA, lactic acid / glycolic acid=90 / 10, molecular weight 120000) was dissolved in THF to make a 0.05wt% solution, and the drug rapamycin was dissolved therein to make a 0.1wt% solution , use a spraying machine to spray, after spraying, dry it under vacuum at 42°C for half an hour to form the polymer layer of the stent, the drug / polymer weight ratio=2:1. The weight of PLGA and rapamycin contained in the stent polymer layer was 13.33 μg / mm pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com