Polymer containing hydroxystyrene on side group, preparation method and application thereof
A technology of hydroxystyrene and styrene derivatives, applied in the field of preparation and polymer polymerization, can solve problems such as poor hydrophilic performance, and achieve the effect of good hydrophilic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
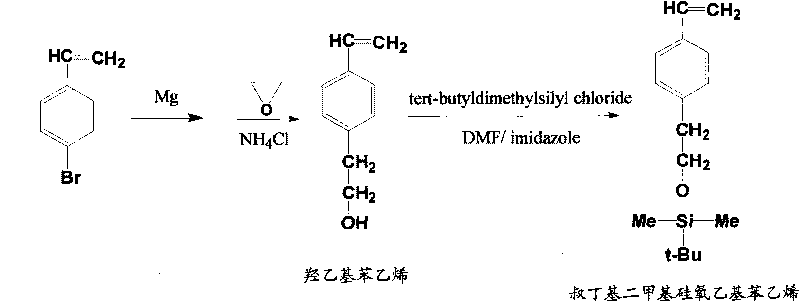
[0043] (1), the preparation of tert-butyldimethylsiloxyethylstyrene
[0044] In a 500mL three-necked flask equipped with magnetic stirring, a constant pressure dropping funnel, and a reflux condenser, add 1.488g (0.062mol) of polished magnesium chips under anaerobic and anhydrous conditions, and 40mL of anhydrous tetrahydrofuran as a solvent. Access to N 2 , Add dropwise a solution of 11.33 g (0.062 mol) of 4-bromostyrene dissolved in 70 mL of tetrahydrofuran, and control the temperature below 40°C. After the dropwise addition, the reaction was continued until the magnesium reaction was complete. The mixture was cooled to -15°C with pre-cooled petroleum ether, and a mixed solution of ethylene oxide (3.83 g, 0.087 mol) and 80 mL of anhydrous tetrahydrofuran was added rapidly. After the addition, the reaction was continued at room temperature for 3 h. The molar ratio between magnesium, 4-bromostyrene and ethylene oxide is 1:1:1.4. At 0°C, add 100mL of ether, slowly add 100mL o...
Embodiment 2
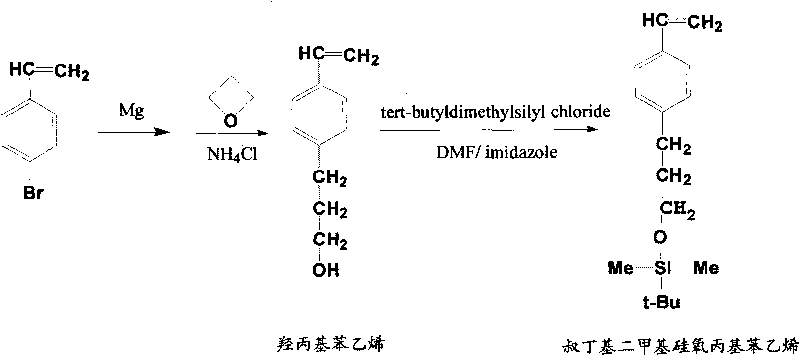
[0053] Cationic polymerization method, the preparation of poly-tert-butyldimethylsiloxypropylstyrene; then, according to the method of embodiment 1 (3), the hydrolysis of poly-tert-butyldimethylsiloxypropylstyrene is obtained Polyhydroxyethylstyrene.
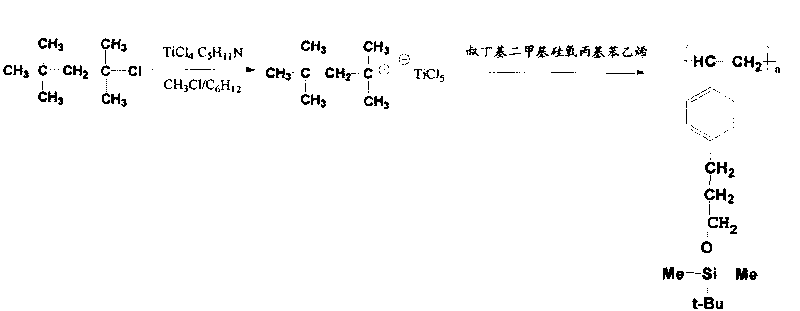
[0054] Cationic polymerization was performed in an inert gas glove box with a low temperature cold bath. At -80°C, add 20 mL of a mixed solvent of cyclohexane / chloromethane (50 / 50 v / v) to the dry polymerization bottle, and then continuously add the initiator TMPCl solution (0.002 mol / L), the third group Separate hexahydropyridine solution (1mL, 0.002mol / L) and co-initiator dichloroethyl aluminum (1mL, 0.036mol / L) solution. The molar ratio of the initiator, the third component and the co-initiator is 1.0:1.0:18, aging for 15 min, and then adding tert-butyldimethylsiloxypropylstyrene (2 mL) for polymerization. After completion, stop the reaction with methanol, precipitate, evaporate the solvent, and dry to constant weight to obt...
Embodiment 3
[0056] Cationic polymerization method, the preparation of the block copolymer of isobutylene and tert-butyldimethylsiloxypropyl styrene; then, according to the method of embodiment 1 (3), poly-tert-butyldimethylsilane Hydrolysis of oxypropyl styrene yields polyisobutylene-b polyhydroxypropyl styrene.
[0057] Cationic polymerization was performed in an inert gas glove box with a low temperature cold bath. At -80°C, add 20 mL of a mixed solvent of cyclohexane / chloromethane (50 / 50 v / v) to the dry polymerization bottle, wherein the volume ratio of tetrahydrofuran to N,N-dimethylformamide is 1: 0.1~10, then continuously add initiator TMPCl solution (0.002mol / L), third component hexahydropyridine solution (1mL, 0.002mol / L), co-initiator dichloroethylaluminum (1mL, 0.036mol / L ) solution. The molar ratio of the initiator, the third component and the co-initiator is 1.0:1.0:18, aging for 15 minutes, then adding isobutylene (2 mL), and after polymerization for 40 minutes, adding tert...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com