Pharmaceutical preparation containing isosorbide mononitrate
A technology of isosorbide dinitrate and drugs, which is applied in the field of medicine, can solve the problems of not being able to know the types and dosages of excipients of preparation compositions, making it difficult to ensure drug safety for patients, and increasing the metabolic burden of the body, so as to avoid multi-dose drug resistance, Delays the effects of multi-dose resistance, reduction in the amount of metabolized drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
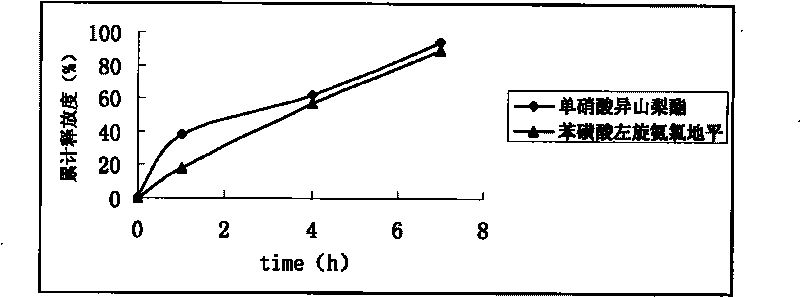
Embodiment 1
[0033] The tablet contains the following ingredients by weight percentage:
[0034] Core composition:
[0035] Isosorbide mononitrate 40g
[0036] L-Amlodipine Besylate 2.5g
[0037] Hypromellose 60g
[0038] Lactose 160g
[0039] 8% polyvinylpyrrolidone K30 ethanol solution
[0041]
[0042] Prepared into 1000 tablets
[0043] Composition of coating liquid:
[0044] Hypromellose 6cp 4g
[0045] PEG-4000 1g
[0046] Talc 1.5g
[0047] Titanium dioxide 1.5g
[0048] Ethanol / water (60 / 40) 100ml
[0049]
[0050] Coating weight gain 3%
[0051] Using conventional techniques, isosorbide mononitrate and levoamlodipine besylate are made into single-layer sustained-release tablets, and then film-coated.
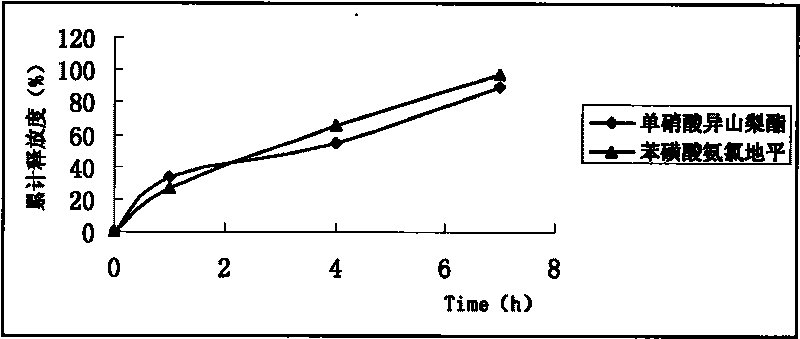
Embodiment 2
[0053] The tablet contains the following ingredients by weight percentage:
[0054] Core composition:
[0055] Isosorbide mononitrate 40g
[0056] Amlodipine besylate 5g
[0057] Hypromellose 72g
[0058] Lactose 80g
[0059] Mannitol 80g
[0060] 8% polyvinylpyrrolidone K30 ethanol solution
[0062]
[0063] Prepared into 1000 tablets
[0064] Composition of coating liquid:
[0065] Hypromellose 6cp 4g
[0066] PEG-4000 1g
[0067] Talc 1.8g
[0068] Titanium dioxide 1.8g
[0069] Ethanol / water (70 / 30) 100ml
[0070]
[0071] Coating weight gain 3%
[0072] Using conventional techniques, isosorbide mononitrate and amlodipine besylate are made into single-layer sustained-release tablets, and then film-coated.
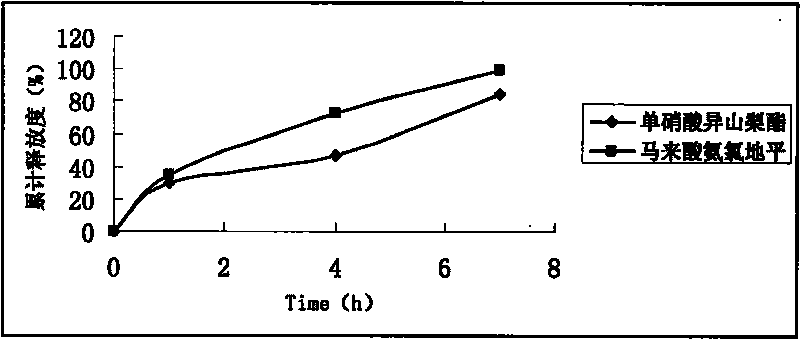
Embodiment 3
[0074] The tablet contains the following ingredients by weight percentage:
[0075] Core composition:
[0076] Isosorbide mononitrate 40g
[0077] Amlodipine maleate 10g
[0078] Hypromellose 80g
[0079] Microcrystalline cellulose 130g
[0080] 5% ethyl cellulose absolute ethanol solution
[0082]
[0083] Prepared into 1000 tablets
[0084] Composition of coating liquid:
[0085] Hypromellose 6cp 4g
[0086] PEG-2000 1g
[0087] Talc 1.5g
[0088] Titanium dioxide 1.5g
[0089] Ethanol / water (60 / 40) 100ml
[0090]
[0091] Coating weight gain 3%
[0092] Using conventional techniques, isosorbide mononitrate and amlodipine maleate are made into single-layer sustained-release tablets, and then film-coated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com