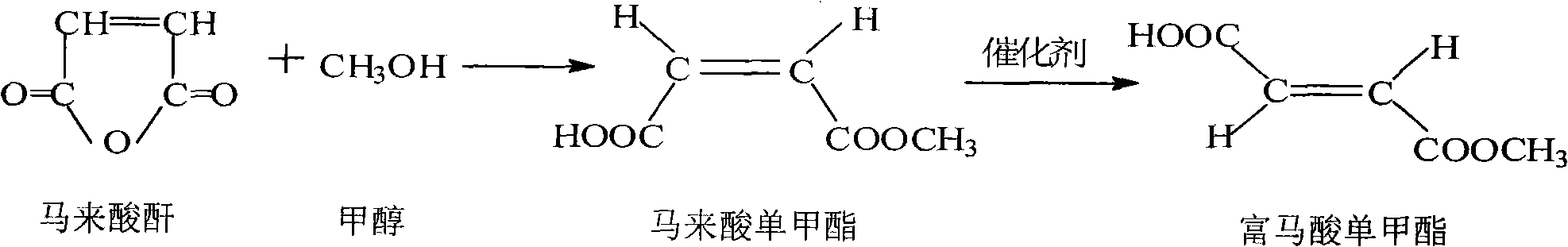
Preparation method of monomethyl fumarate
A technology of monomethyl fumarate and monomethyl maleate, which is applied in the field of preparation of monomethyl fumarate, can solve the problems of increasing production costs and unfavorable industrial production, and achieve energy-saving reaction time and environmental protection performance Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of fumaryl chloride
[0028] Add 58.0g (0.5mol) of fumaric acid and a few drops of catalyst in the reaction flask equipped with an acid gas recovery device, and then add 178.5g (1.5mol, excess) of SOCl 2 . Heat to reflux until the fumaric acid is completely reacted. Atmospheric distillation recovered excess thionyl chloride, and then vacuum distillation to collect fractions at 80-82°C / 0.09MPa to obtain 71 g of light yellow liquid, namely fumaryl chloride, with a yield of 92%.
[0029] Preparation of monomethyl fumarate
[0030] Put 49g (0.5mol) of maleic anhydride and 12.8g (0.4mol) of anhydrous methanol into the reaction flask, stir and react at room temperature, the temperature of the system automatically rises to about 35°C, react for 30 to 40 minutes, and the maleic anhydride is completely dissolved. Add 6.4 g (0.2 mol) of anhydrous methanol, and heat to 55° C. in a water bath to continue the reaction for 30 minutes.
[0031] Heat the above reaction ...
Embodiment 2
[0033] Preparation of monomethyl fumarate
[0034] Put 49g (0.5mol) of maleic anhydride and 12.8g (0.4mol) of anhydrous methanol into the reaction flask, stir and react at room temperature, the temperature of the system automatically rises to about 35°C, react for 30 to 40 minutes, and the maleic anhydride is completely dissolved. Add 6.4 g (0.2 mol) of anhydrous methanol, and heat to 55° C. in a water bath to continue the reaction for 30 minutes.
[0035] Add a certain amount of ethyl acetate to the above reaction system, heat, adjust the temperature of the hot bath to 90°C, add 3.06g (0.02mol) fumaryl chloride to the reaction system, and the temperature of the reaction system rises sharply to 90°C. After reacting for 2 minutes, crystals began to separate out in the solution, and solidified quickly. Under the condition of heat preservation, the temperature of the reaction system will further increase to 96°C. After 10 minutes of heat preservation, the temperature of the system...
Embodiment 3
[0037] Preparation of monomethyl fumarate
[0038] Put 98g (1.0mol) of maleic anhydride and 28.8g (0.9mol) of anhydrous methanol into the reaction flask, stir and react at room temperature, the temperature of the system automatically rises to about 35°C, react for 30-40 minutes, and the maleic anhydride is completely dissolved. Add 6.4 g (0.2 mol) of anhydrous methanol, and heat to 55° C. in a water bath to continue the reaction for 30 minutes.
[0039] After adding a certain amount of ethyl acetate to the above reaction system and heating to a system temperature of 85° C., 1.53 g (0.01 mol) of fumaryl chloride was added to the reaction system, and the temperature of the reaction system rose sharply to 90° C. After reacting for 2 minutes, crystals began to separate out in the solution, and solidified quickly. The reaction temperature of the system was further increased to 95°C, and after 10 minutes of heat preservation, the system temperature began to decrease, and the temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com