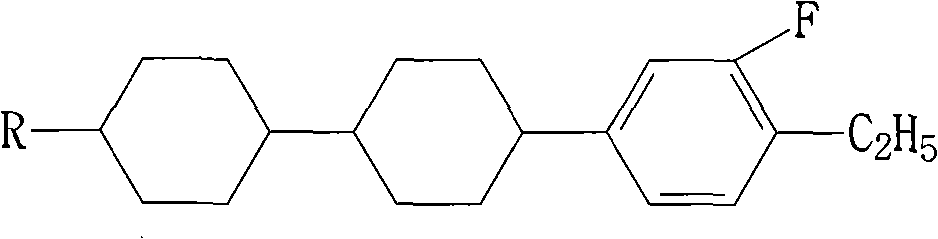
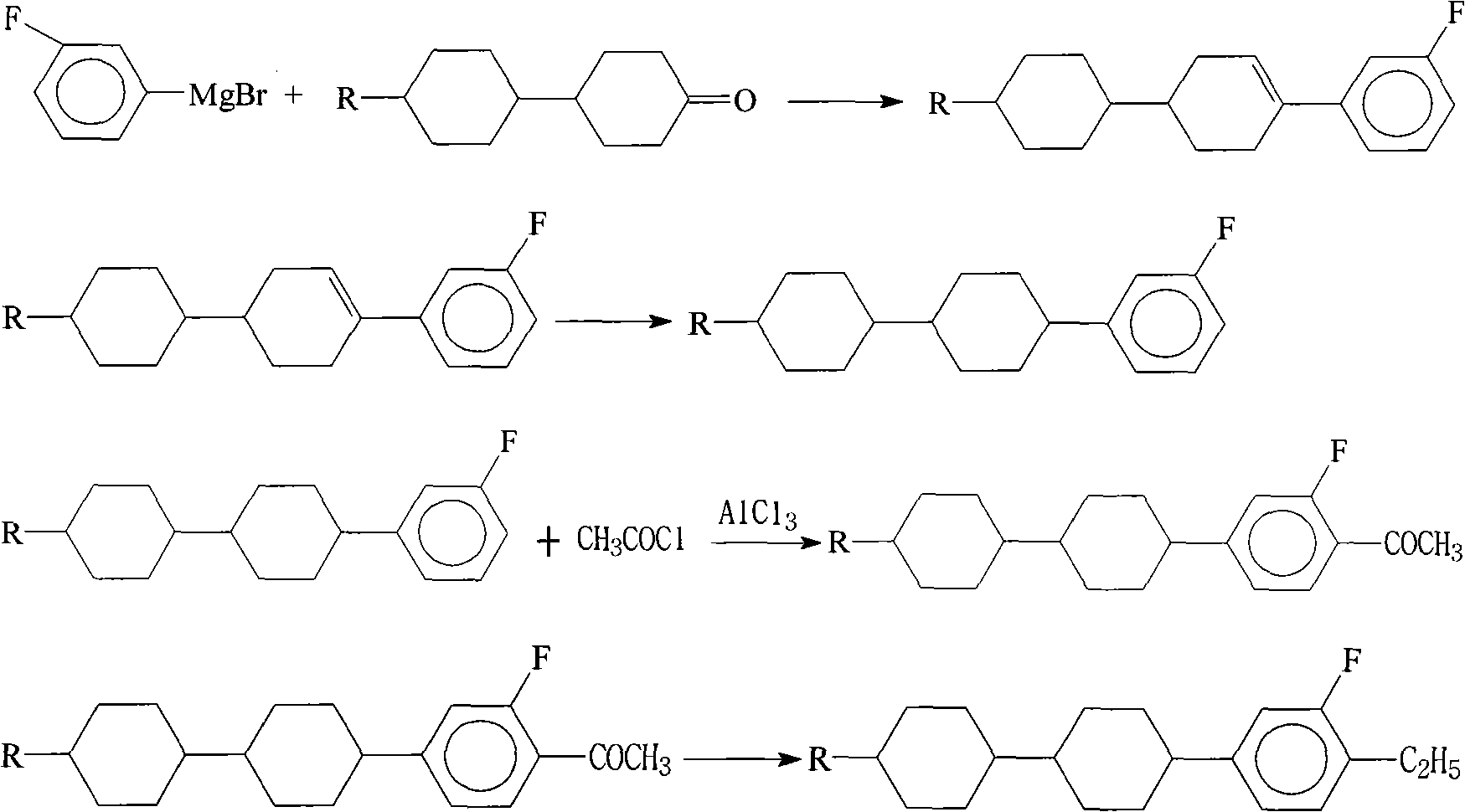
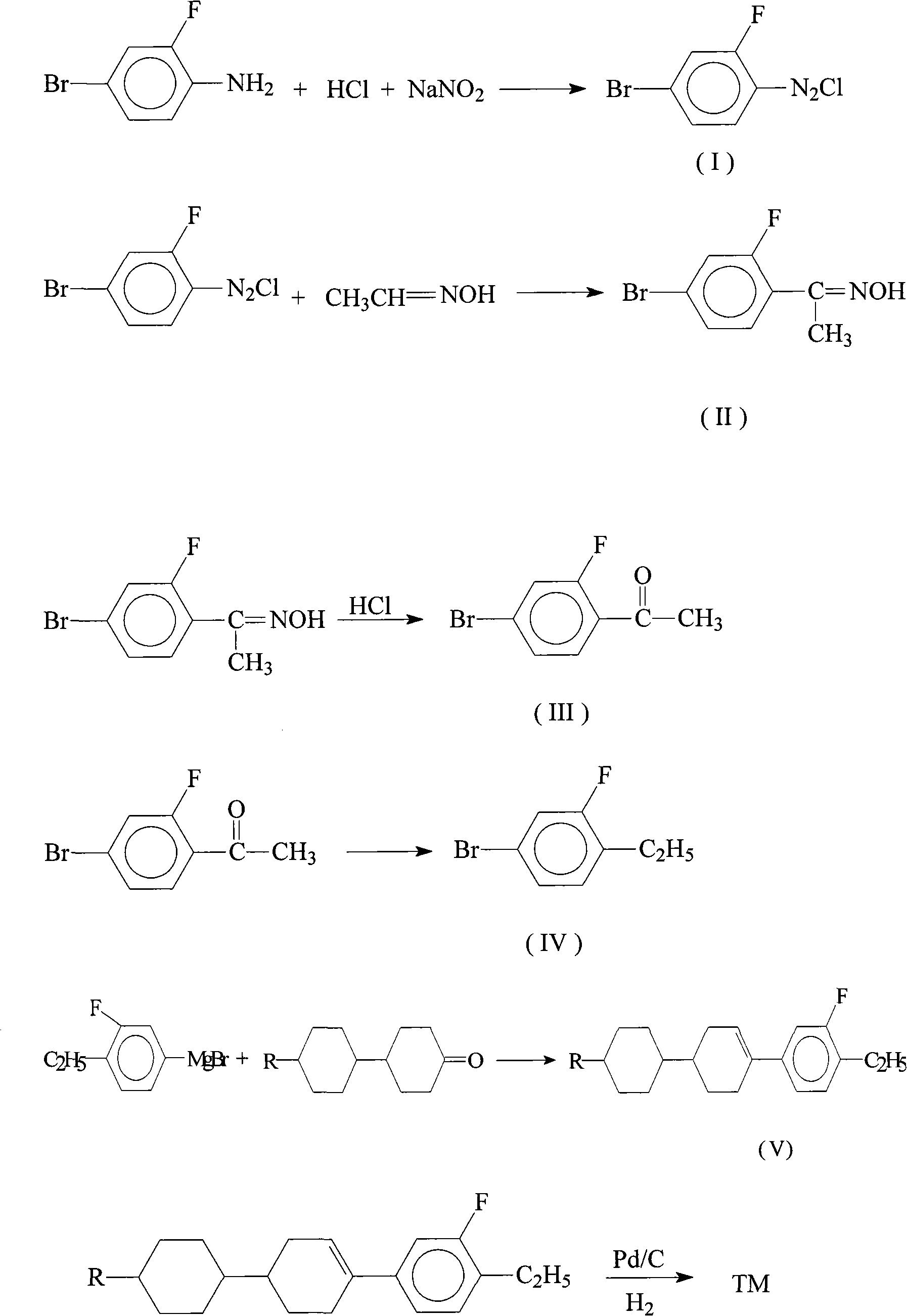Method for synthesizing dicyclohexyl o-fluoroethylbenzene liquid crystal compound
A liquid crystal compound, bicyclohexyl technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve the problem of high cost, achieve the effect of improving yield and purity, simple operation and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 43ml of 11.2mol / L concentrated hydrochloric acid and 120ml of water to a 1L three-necked flask, slowly add 95g of o-fluoro-p-bromoaniline dropwise under heating and stirring, heat until dissolved, cool, add 45ml of hydrochloric acid under stirring, keep in an ice-water bath for 1.5 hours, use dropwise Add a funnel below the liquid surface and slowly add 60ml of aqueous solution containing 35g of sodium nitrite, and continue stirring for 1 hour after the dripping is complete. Sodium acetate aqueous solution adjusts the pH value of the reaction system to be approximately equal to 5, quickly filters out a small amount of deep red solid in the solution, and puts the light yellow diazonium salt solution in the refrigerator for use.
Embodiment 2
[0022] Add 200ml of water, 15g of copper sulfate pentahydrate, and 42g of acetic acid to the there-necked flask, stir and dissolve, then add 28.2g of 10% aqueous solution of acetaldehyde oxime, 1.Sg of sodium sulfite and stir for 10 minutes, then adjust the pH value of the system to neutral with sodium acetate , lower the temperature to below 10°C, slowly add the diazonium salt solution below the liquid level with a dropping funnel under rapid stirring, continue stirring for 1 hour, add 240ml of toluene, stand to separate the organic layer, and obtain compound (II).
Embodiment 3
[0024] Add the organic phase obtained in the above example into a three-necked flask, add 240ml of water and 300ml of 11.2mol / L concentrated hydrochloric acid, heat to reflux for 3 hours, cool to room temperature, separate the organic phase, and wash the organic phase with 200ml of 5% aqueous sodium carbonate To neutrality, the aqueous phase was extracted with 100ml of ether, then dried with 50g of anhydrous magnesium sulfate, and rectified under reduced pressure to obtain compound (III).
[0025] The yield is 73%; the gas phase purity is 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com