Stable compound dimeticone hydrotalcite suspension
A technology of compound simethicone and aluminum magnesium carbonate, which is applied in the directions of aluminum/calcium/magnesium active ingredients, liquid delivery, pharmaceutical formulations, etc., can solve problems such as environmental pollution, complex formulations and processes, and no manufacturer declaration, and reduce production. cost, reduce environmental pollution and toxicity, and improve the effect of safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
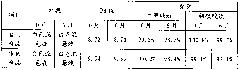
[0032] 1. Prescription:
[0033] Aluminum magnesium carbonate 100g
[0034] Simethicone 25g
[0035] CMC-Na 20g
[0036] Polyoxyethylene (40) Hydrogenated Castor Oil 15g
[0037] Purified water up to 1000ml
[0038] 2. Process steps:
[0039] (1) CMC-Na was dissolved in 400ml purified water to obtain solution I;
[0040] (2) Get 300ml of solution I, add aluminum magnesium carbonate, pass through a colloid mill, make its average particle diameter be below 50 μm, collect the colloid mill effluent to obtain solution II;
[0041] (3) Take 100ml of solution I, add simethicone, polyoxyethylene (40) hydrogenated castor oil, pass through a colloid mill to make the average particle size below 50 μm, collect the colloid mill effluent to obtain solution III, add solution II, Add purified water to 1000ml;
[0042] (4) Filling, specification 30ml / bottle.
Embodiment 2
[0044] 1. Prescription:
[0045] Aluminum magnesium carbonate 100g
[0046] Simethicone 25g
[0047] CMC-Na 12g
[0048] Polyoxyethylene (40) Hydrogenated Castor Oil 10g
[0049] Micronized silica gel 5g
[0050] Methylparaben 1.8g
[0051] Appropriate amount of hydrochloric acid to adjust PH
[0052] Purified water up to 1000ml
[0053] 2. Process steps:
[0054] (1) CMC-Na was dissolved in 700ml of water to obtain solution I;
[0055] (2) Methylparaben was dissolved in 100ml of purified water at a temperature of 80°C, and kept warm for later use to obtain solution II;
[0056] (3) Get solution I 300ml, add aluminum magnesium carbonate, cross colloid mill, make its average particle diameter be below 50 μ m, collect colloid mill effluent, obtain solution III;
[0057] (4) Take 400ml of solution I, add simethicone, micropowder silica gel, polyoxyethylene (40) hydrogenated castor oil, pass through a colloid mill to make the average particle size below 50 μm, collect the...
Embodiment 3
[0080] 1. Prescription:
[0081] Aluminum magnesium carbonate 50g
[0082] Simethicone 12.5g
[0083] CMC-Na 12g
[0084] Polyoxyethylene (40) hydrogenated castor oil 8g
[0085] Micronized silica gel 4g
[0086] Methylparaben 1.8g
[0087] Appropriate amount of hydrochloric acid to adjust PH
[0088] Purified water up to 1000ml
[0089] 2. Process steps:
[0090] (1) CMC-Na was dissolved in 500ml of water to obtain solution I;
[0091] (2) Methylparaben was dissolved in 200ml of purified water at a temperature of 80°C, and kept warm for later use to obtain solution II;
[0092] (3) Get solution I 300ml, add aluminum magnesium carbonate, cross colloid mill, make its average particle diameter be below 50 μ m, collect colloid mill effluent, obtain solution III;
[0093] (4) Take 200ml of solution I, add simethicone, micropowder silica gel, polyoxyethylene (40) hydrogenated castor oil, pass through a colloid mill to make the average particle size below 50 μm, collect the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com