Porous calcium phosphate microsphere with medicinal controlled release function, preparation method and application thereof
A technology for controlled release of porous calcium phosphate and drugs, applied in the field of porous calcium phosphate microspheres and preparation, can solve the problems of inability to achieve filling, bone cement plasticity and fluidity limitation, etc. The effect of good liquidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: composition is 10Li 2 O-10CaO-80B 2 o 3 (wt%) preparation of porous calcium phosphate microspheres.
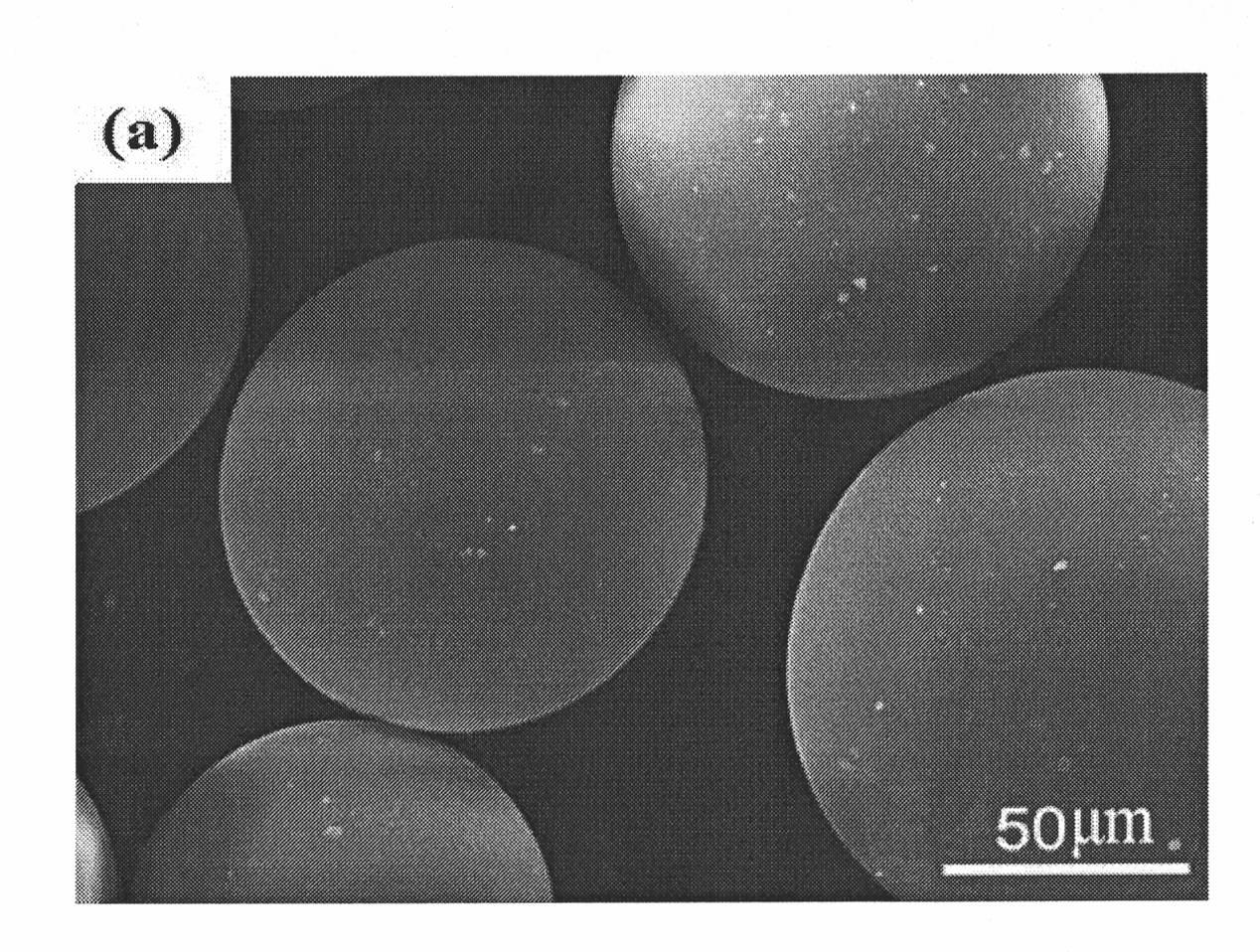
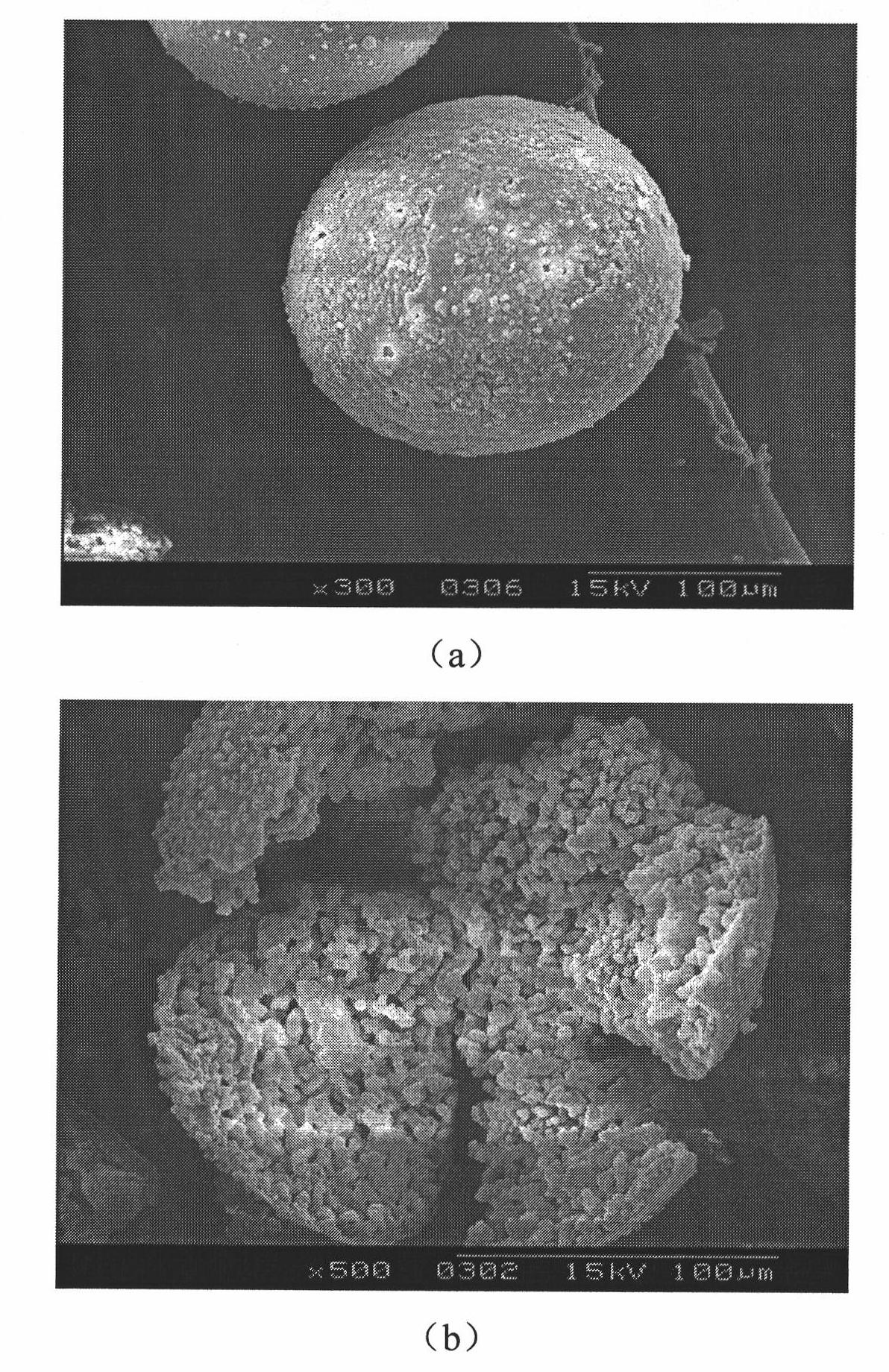
[0021] The composition of 10Li was prepared by high temperature melting method 2 O-10CaO-80B 2 o 3 (wt%) borate glass, the glass is crushed and sieved to obtain glass particles between 97 μm and 154 μm. After repeated spheroidization of glass particles by flame spraying method, the obtained borate glass microspheres were collected.
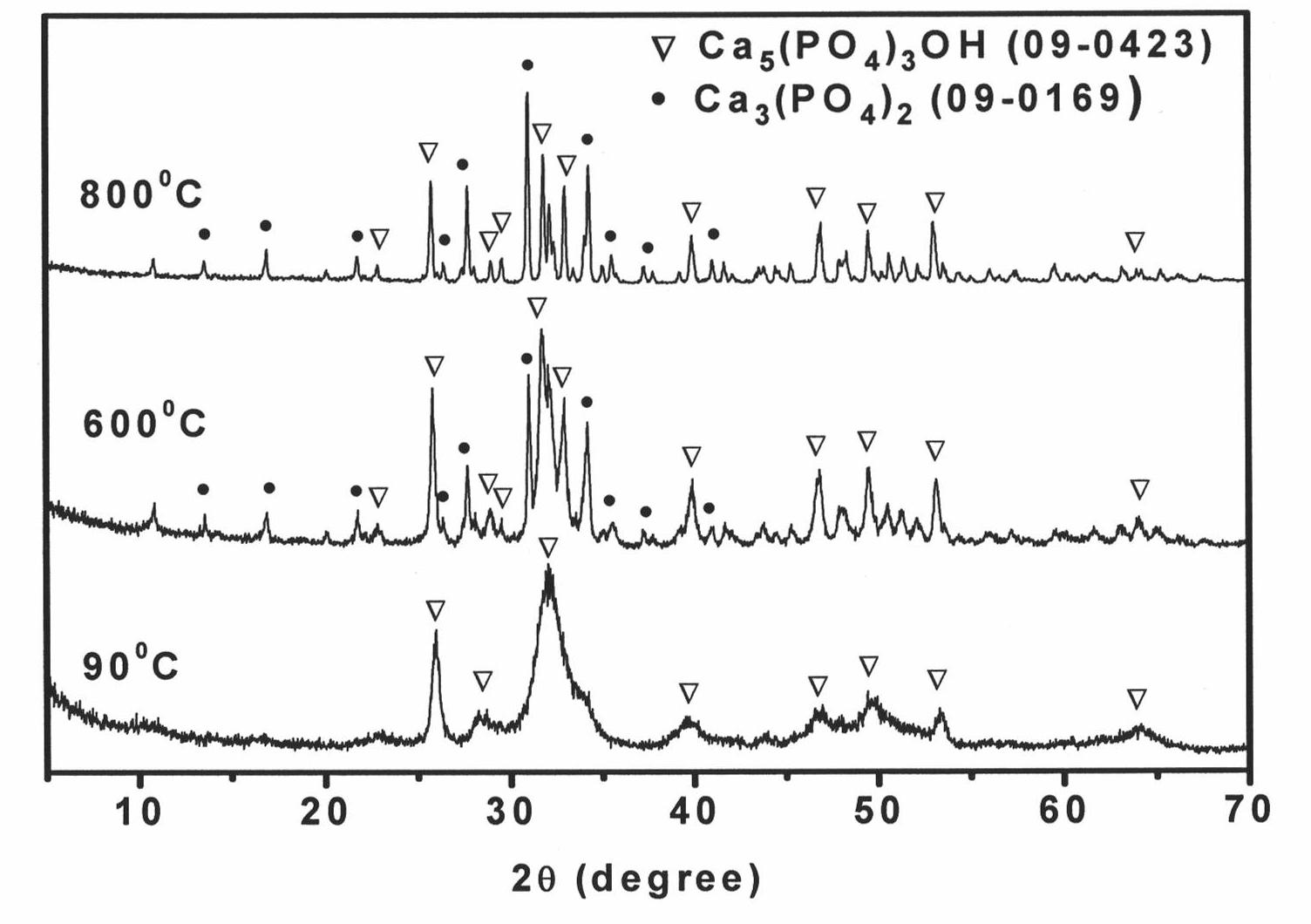
[0022] Prepare K with a concentration of 0.1mol / L 2 HPO 4 The solution is used as a reaction soaking solution, and the pH value of the solution is adjusted to be greater than 9.0 by a pH meter. Soak 1 g of the borate glass microspheres obtained above in 100 mL of K 2 HPO 4 solution, put it in a constant temperature box at 37°C, soak it for 7 days, and then take it out. Remove the soaking solution, rinse with deionized water three times, and dry in an oven at 90°C for 24 hours. The dried microspheres were calcined a...
Embodiment 2
[0023] Embodiment 2: composition is 7.5Li 2 O-40CaO-52.5B 2 o 3 (wt%) preparation of porous calcium phosphate microspheres.
[0024] The composition is 7.5Li prepared by high temperature melting method 2 O-40CaO-52.5B 2 o 3 (wt%) borate glass, the glass is crushed and sieved to obtain glass particles between 97 μm and 154 μm. After repeated spheroidization of glass particles by flame spraying method, the obtained borate glass microspheres were collected.
[0025] Prepare K with a concentration of 0.25mol / L 2 HPO 4 The solution is used as a reaction soaking solution, and the pH value of the solution is adjusted to be greater than 9.0 by a pH meter. Soak 1 g of the borate glass microspheres obtained above in 100 mL of K 2 HPO 4 solution, put it in a constant temperature box at 60°C, take it out after soaking for 7 days. Remove the soaking solution, rinse three times with deionized water, and dry in an oven at 90°C for 24 hours. The dried microspheres were calcined at...
Embodiment 3
[0026] Embodiment 3: rifampicin medicine is composed of 10Li 2 O-10CaO-80B 2 o 3 (wt%) loading and release in porous calcium phosphate microspheres.
[0027] Loading rifampicin into porous calcium phosphate microspheres by pressure osmosis. Weigh 50 mg of porous microspheres, put into a clean weighing bottle (or small test tube), and pour 10 ml of saturated rifampicin solution. Put it into a vacuum drying oven and keep the vacuum degree <0.085MPa to eliminate the gas in the pores of the microspheres, and make the drug rifampicin diffuse into the pores inside the microspheres through the mechanism of concentration diffusion under the action of pressure. The sample was taken out after 5 min.
[0028] Drug release: Pour 50 mL of PBS solution (0.01 mol / L, pH=7.4) into a plastic bottle, and add the drug-loaded microspheres into the PBS solution. Put the plastic bottle into a constant temperature water bath at 37°C, and slowly release the drug while maintaining vibration. When...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com