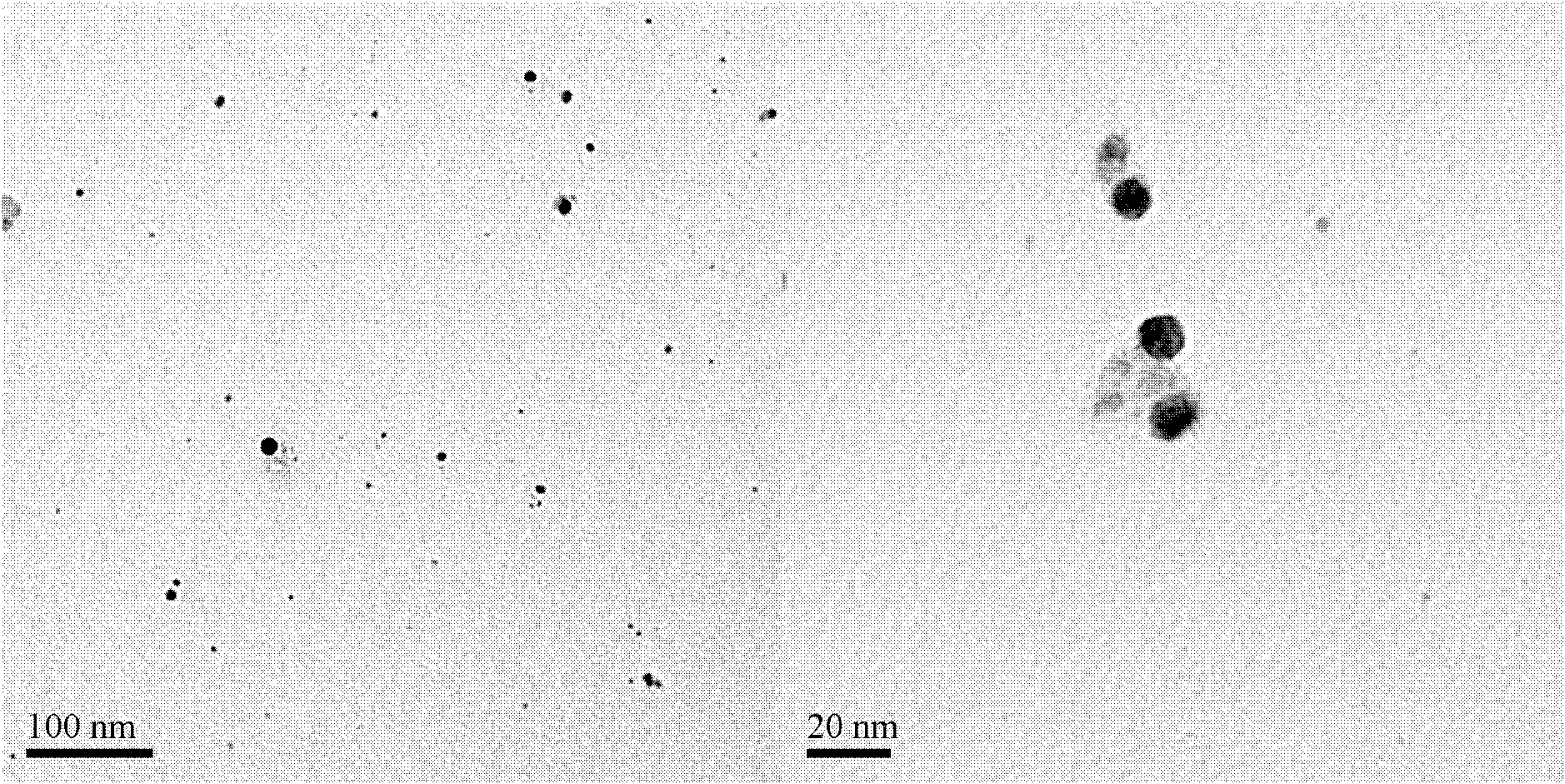
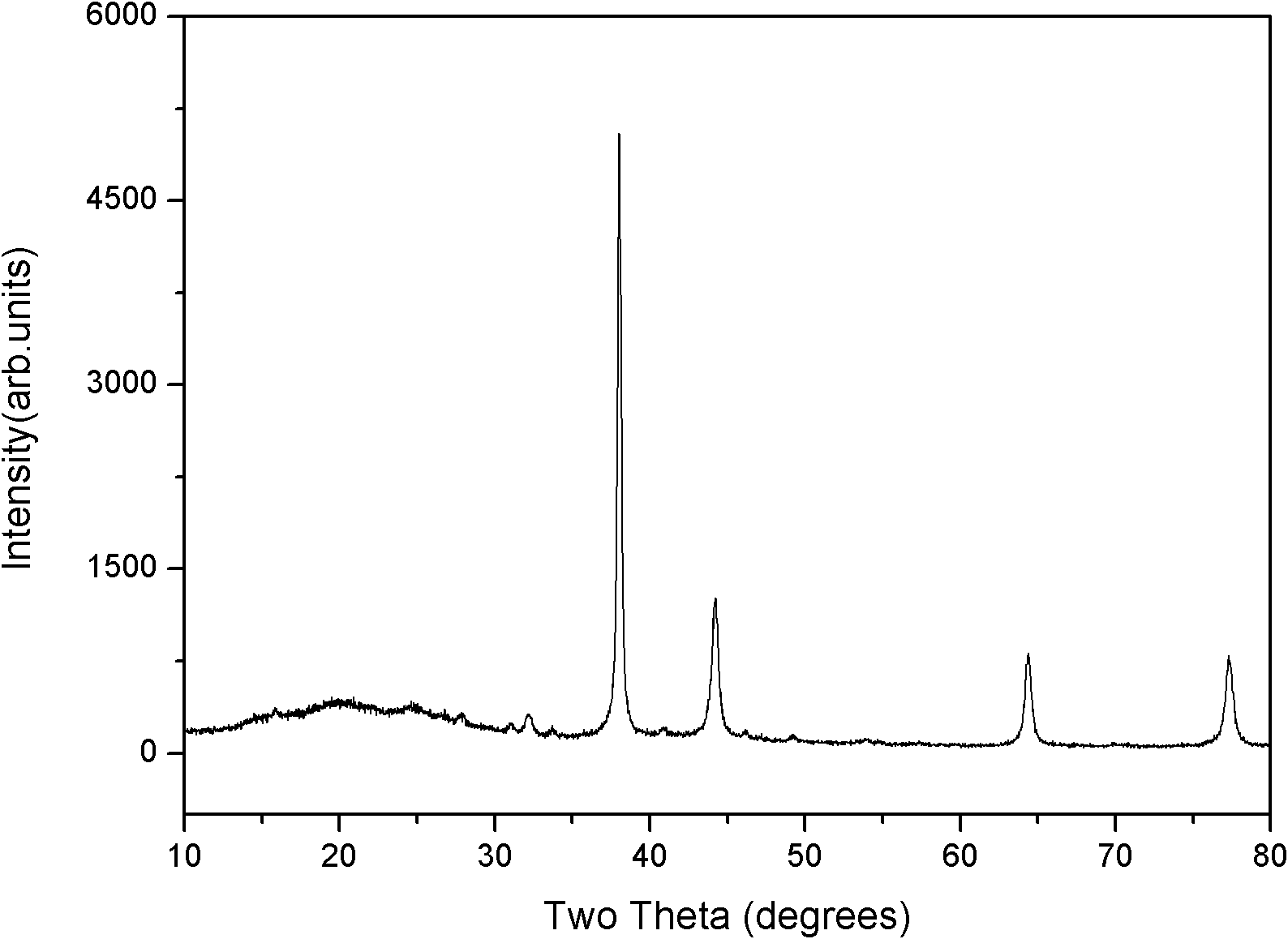
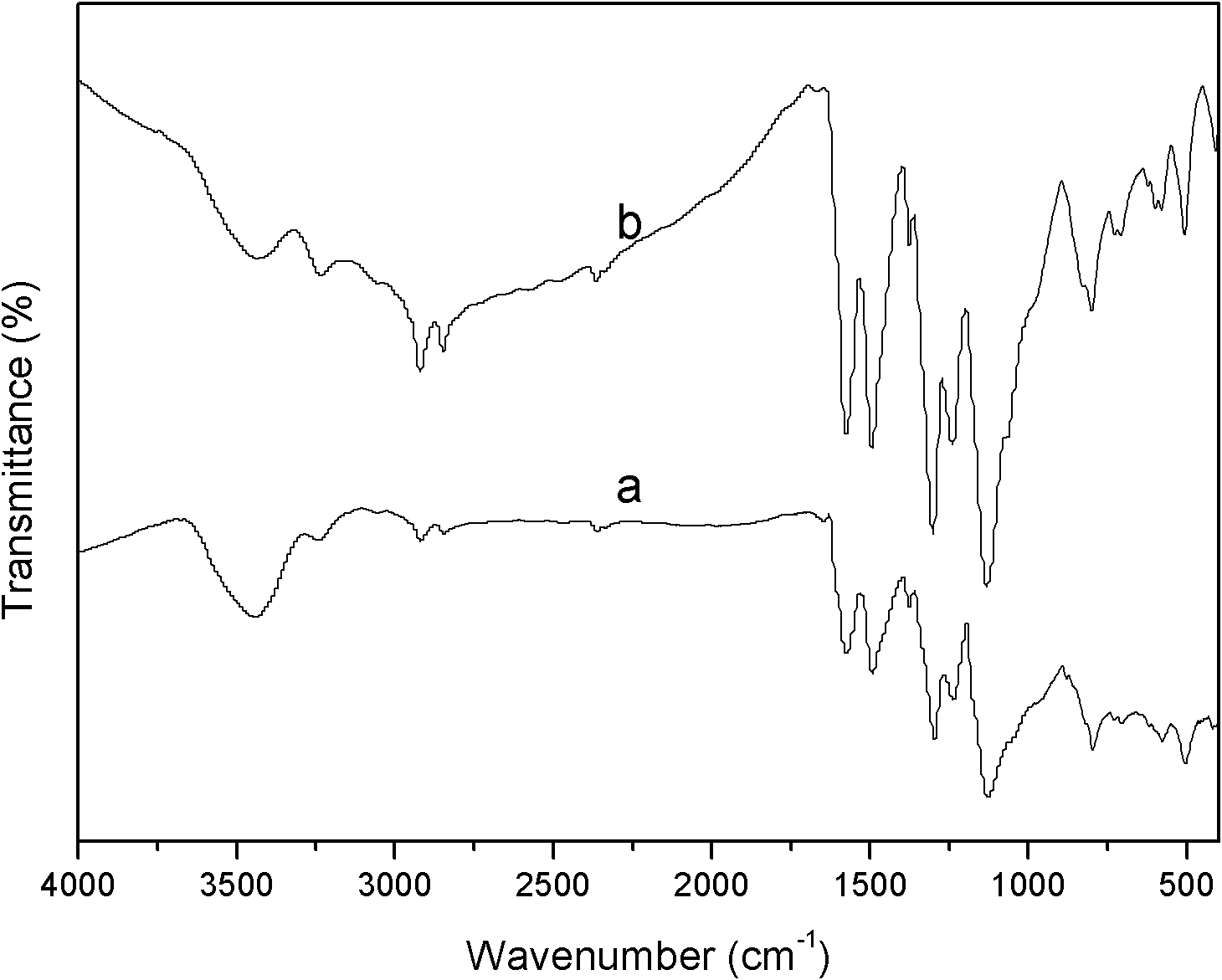
Polyaniline/silver conductive nanocomposite material and preparation method thereof
A composite material, conductive nanotechnology, applied in non-metallic conductors, organic material conductors and other directions, can solve problems that have not been reported in the literature, and achieve the effects of inhibiting agglomeration, uniform morphology, thermal performance and processability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: used silver nitrate (analytically pure), its main component (%) is: AgNO 3 ≥99.8%, water insoluble ≤0.005%, chloride ≤0.001%, sulfate ≤0.004%, Fe≤0.0004%, Cu≤0.001%, Pb≤0.001%, hydrochloric acid non-precipitate ≤0.02%.
[0032] Measure 6ml of 0.5M silver nitrate solution prepared in advance into a beaker, add 5.35g of n-hexanol, 150ml of cyclohexane and 2.16g of sodium lauryl sulfate, place it in ultrasonic cleaning, and ultrasonically disperse it to form a translucent reverse glue Beam solution A. Weigh a certain amount of aniline and dissolve it in 1M nitric acid solution, configure 0.5M aniline / nitric acid solution, measure 6ml of aniline / nitric acid solution in a beaker, add 5.35g n-hexanol, 150ml cyclohexane and 2.16g dodecyl Sodium sulfate, as above, prepare an equal volume of reverse micellar solution B. Mix the reverse micellar solutions A and B, and magnetically stir for 10 minutes to make them evenly mixed. Cover the mouth of the beaker with a...
Embodiment 2
[0035]Measure 6ml of 0.2M silver nitrate solution prepared in advance into a beaker, add 5.35g of n-hexanol, 150ml of cyclohexane and 2.16g of sodium lauryl sulfate, and ultrasonically disperse to form reverse micellar solution A. Prepare 0.2M aniline / nitric acid solution, measure 4ml of aniline / nitric acid solution, and prepare an equal volume of reverse micellar solution B as above. Mix the reverse micellar solutions A and B, and magnetically stir to make them evenly mixed. Cover the mouth of the beaker with a watch glass, place the reaction system in a fume hood, and keep the purple light lamp at a vertical distance of 10 cm from the liquid surface. Turn on the purple light, and magnetically stir the reaction at room temperature for 20 h. Add methanol, stir for 3h to break the emulsion, filter and wash until the filtrate is colorless and foam-free. The filter cake was dispersed into 4M hydrochloric acid solution, stirred for 2 h for re-doping, then centrifuged and washed ...
Embodiment 3
[0039] Measure 6ml of 0.5M silver nitrate solution prepared in advance into a beaker, add 5.35g of n-hexanol, 150ml of cyclohexane and 2.16g of sodium lauryl sulfate, and ultrasonically disperse to form reverse micellar solution A. Prepare 0.5M aniline / nitric acid solution, measure 4ml of aniline / nitric acid solution in a beaker, add 5.35g n-hexanol, 150ml cyclohexane and 2.16g sodium dodecyl sulfate, the same as the reverse micellar solution B configured above. Mix the reverse micellar solutions A and B, and magnetically stir to make them evenly mixed. Cover the mouth of the beaker with a watch glass, place the reaction system in a fume hood, and keep the purple light lamp at a vertical distance of 10 cm from the liquid surface. Turn on the purple light, and magnetically stir the reaction at room temperature for 20 h. Add methanol, stir for 3h to break the emulsion, filter and wash until the filtrate is colorless and foam-free. The filter cake was dispersed into 4M sulfuric...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| power | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com