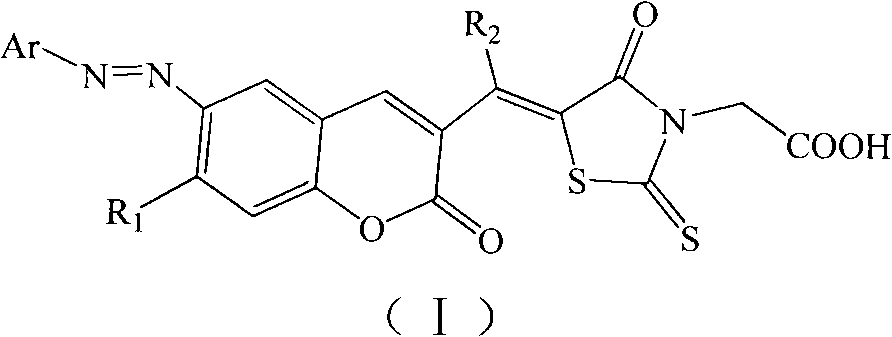
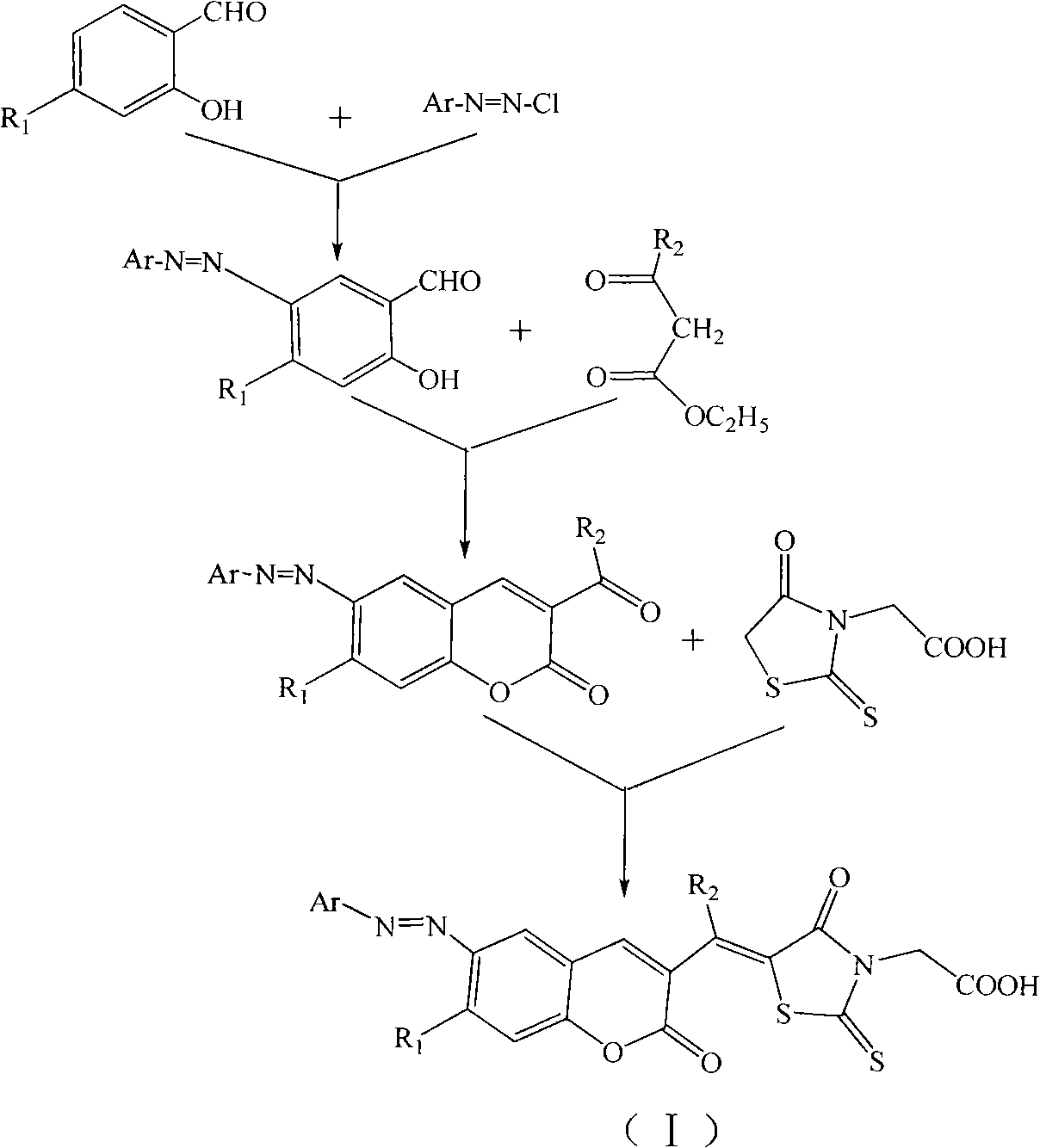
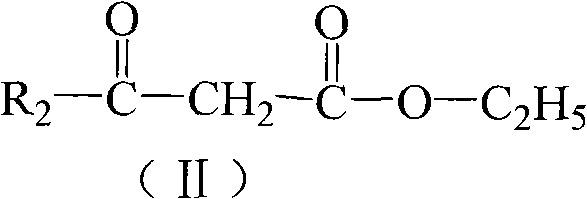Organic dye and preparation method and application thereof
An organic dye, selected technology, applied in the direction of organic dyes, organic chemistry, azo dyes, etc., can solve the problems of insufficient spectral response range, cumbersome preparation process, high cost, etc., and achieve easy preparation and high product yield And the effect of high purity and high degree of commercialization of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 organic dye (Ⅰ-1)
[0030]
[0031] Step 1 In a four-necked flask equipped with a stirrer, use 130 ml of hydrogen bromide glacial acetic acid solution to heat up to 30-40°C to dissolve 0.02 mole of 4-dianilinoaniline, and then add 1.4 grams of it in batches at a temperature of 0-8°C Sodium nitrite reacts with diazonium. After 1 hour, the diazonium solution was poured into the glacial acetic acid solution of salicylaldehyde to carry out the azo reaction. The azo reaction product system was poured into 100 ml of water, and the precipitate was collected. The filter cake was recrystallized with N,N-dimethylformamide / ethanol (volume ratio=1:1), and dried in vacuo to obtain 6.2 grams of yellow powder 5-(4-dianilinophenylazo)salicylaldehyde , yield 79%. The melting point is 166-167°C.
[0032] h 1-NMR (DMSO-d 6 , δ): 5.32 (1H), 6.65-8.18 (m, 17H), 10.03 (s, 1H).
[0033] Elemental Analysis: C 25 h 19 N 3 o 2 Found (calculated): C76...
Embodiment 2
[0043] The preparation of embodiment 2 organic dyes (I-2)
[0044]
[0045] According to the preparation method and steps of Example 1, the 4-dianilinoaniline in Step 1 of the Example was replaced with aniline to prepare a bright orange-red organic dye (I-2), with a melting point of 237-239°C.
[0046] The characteristic UV-visible light absorption peak λmax=474nm and the characteristic fluorescence emission spectrum peak λmax=547nm of the organic dye (I-2) in N,N-dimethylformamide solution.
Embodiment 3
[0047] The preparation of embodiment 3 organic dyes (I-3)
[0048]
[0049] According to the preparation method and steps of Example 1, the 4-dianilinoaniline in step 1 in the example was replaced with 4-dimethylaminoaniline to prepare bright maroon organic dye (I-3), with a melting point of 251-252°C .
[0050] Organic dye (I-3) has UV-visible light absorption characteristic peak λmax=551nm and fluorescence emission spectrum characteristic peak λmax=634nm in N,N-dimethylformamide solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com