Organic second-order non-linear optical chromophore with D-Pi-A structure and decorated by tree-like group, synthesizing method and application thereof
A second-order nonlinear and dendritic technology, which is applied in the field of organic second-order nonlinear optical chromophores and their synthesis, can solve the problems of small electro-optical coefficients, inability to meet the requirements of deviceization, and low polarization efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
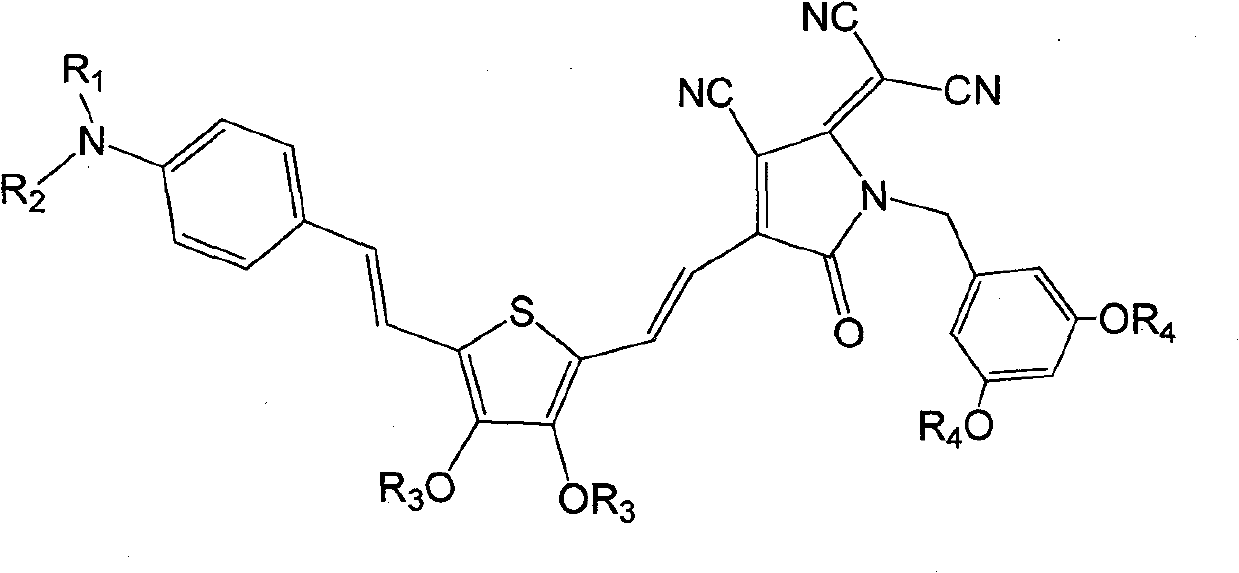
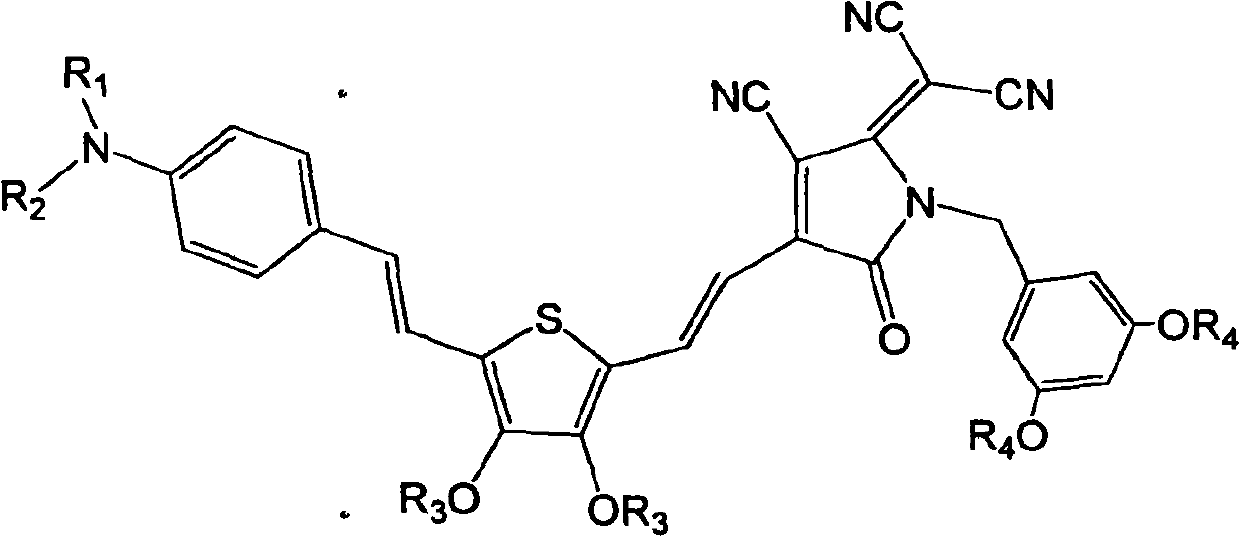
[0041] Synthesis of dendrimer-modified chromophores with D-π-A structure as shown below
[0042]
[0043] The synthetic route is as follows:
[0044]
[0045] Among them, Me is methyl, PPh 3 is triphenylphosphine, TBDMSCl is tert-butyldimethylsilyl chloride, and TCP is a tricyanopyrroline electron acceptor, and its structure is:
[0046]
[0047] The synthesis method is:
[0048] 1) Synthesis of Compound 2
[0049] Get 4g (0.0064mol) bromo-3,4-dihexyloxythienyltriphenylphosphine, 1.9g (0.0064mol) acetyl protected 4-(N,N-dihydroxyethyl)aminobenzaldehyde Electron donor, 4.6g (0.192mol) sodium hydride (NaH) mixed in 100ml of anhydrous ether solvent, stirred at 50 ° C for 18 hours, the reaction product was poured into 200ml of ice water, separated, ether extracted the water phase, combined The organic phase was dried with anhydrous magnesium sulfate, filtered, and the ether was removed by rotary evaporation to obtain a brown-red viscous substance, which was separated ...
Embodiment 2
[0062] Synthesis of dendrimer-modified chromophores with D-π-A structure as shown below
[0063]
[0064] The synthetic route is as follows:
[0065]
[0066] Among them, PPh 3 It is triphenylphosphine, TCP is tricyanopyrroline electron acceptor, its structure is
[0067]
[0068] The synthesis method is:
[0069] 1) Synthesis of Compound 2
[0070] Get 4g (0.0064mol) bromo-3,4-dihexyloxythienyltriphenylphosphine, 1.1g (0.0064mol) 4-(N,N-diethyl)aminobenzaldehyde electron donor, 2.3 g (0.096mol) of sodium hydride (NaH) was mixed in 100ml of anhydrous ether solvent, and after stirring at 20°C for 50 hours, the reaction product was poured into 200ml of ice water, separated, the aqueous phase was extracted with ether, the organic phase was combined, and Dry the organic phase with anhydrous magnesium sulfate, filter, and remove ether by rotary evaporation to obtain a red sticky substance, which is separated by column chromatography (stationary phase is 200-300 mesh si...
Embodiment 3
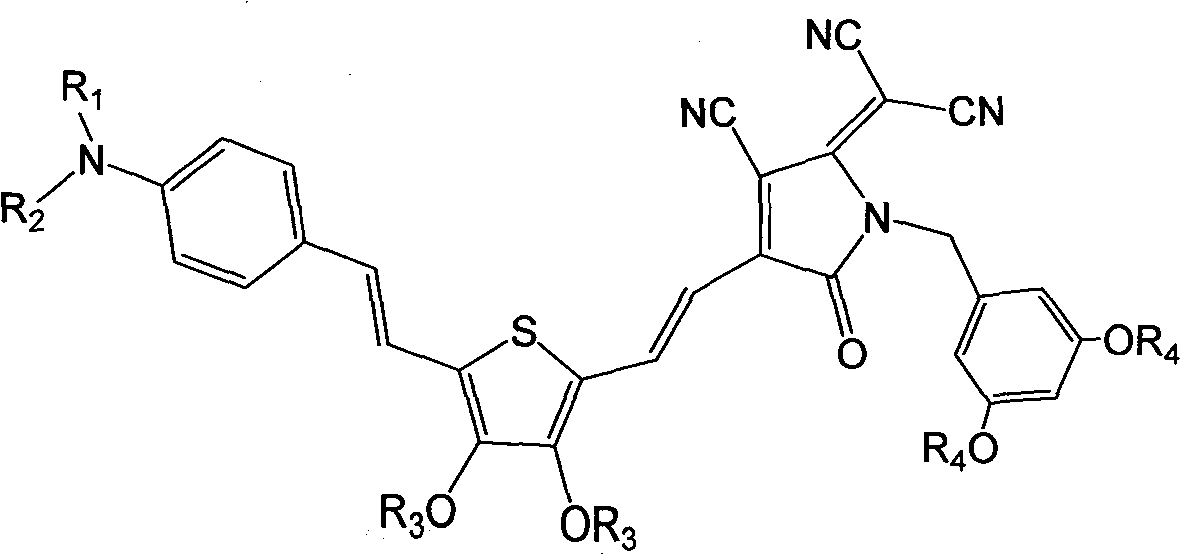
[0078] Synthesis of dendrimer-modified chromophores with D-π-A structure as shown below
[0079]
[0080] The synthetic route is as follows:
[0081]
[0082] Among them, Me is methyl, PPh 3 is triphenylphosphine, TBDMSCl is tert-butyldimethylsilyl chloride, and TCP is tricyanopyrroline electron acceptor, its structure is
[0083]
[0084] The synthesis method is:
[0085] 1) Synthesis of Compound 2
[0086] Take 4g (0.0068mol) of bromo-3,4-dibutoxythienyltriphenylphosphine, 1.5g (0.0068mol) of acetyl-protected 4-(N-methyl, N-hydroxyethyl)amino Benzaldehyde electron donor and 4.1g (0.17mol) sodium hydride (NaH) were mixed in 100ml of anhydrous ether solvent, stirred at 40°C for 24 hours, the reaction product was poured into 200ml of ice water, separated, and the aqueous phase was extracted with ether , combine the organic phases, dry the organic phases with anhydrous magnesium sulfate, filter, and remove ether by rotary evaporation to obtain a brown-red viscous su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electro-optic coefficient | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com