Prokaryotic expression vector of dihydroxyacetone kinase and construction method and applications thereof
A prokaryotic expression and carrier technology, which is applied in the field of prokaryotic expression vectors for high-efficiency expression of dihydroxyacetone kinase protein, can solve problems such as complicated operation process, lack of research and purification, and inconvenient reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation and detection of Pichia pastoris genomic DNA:
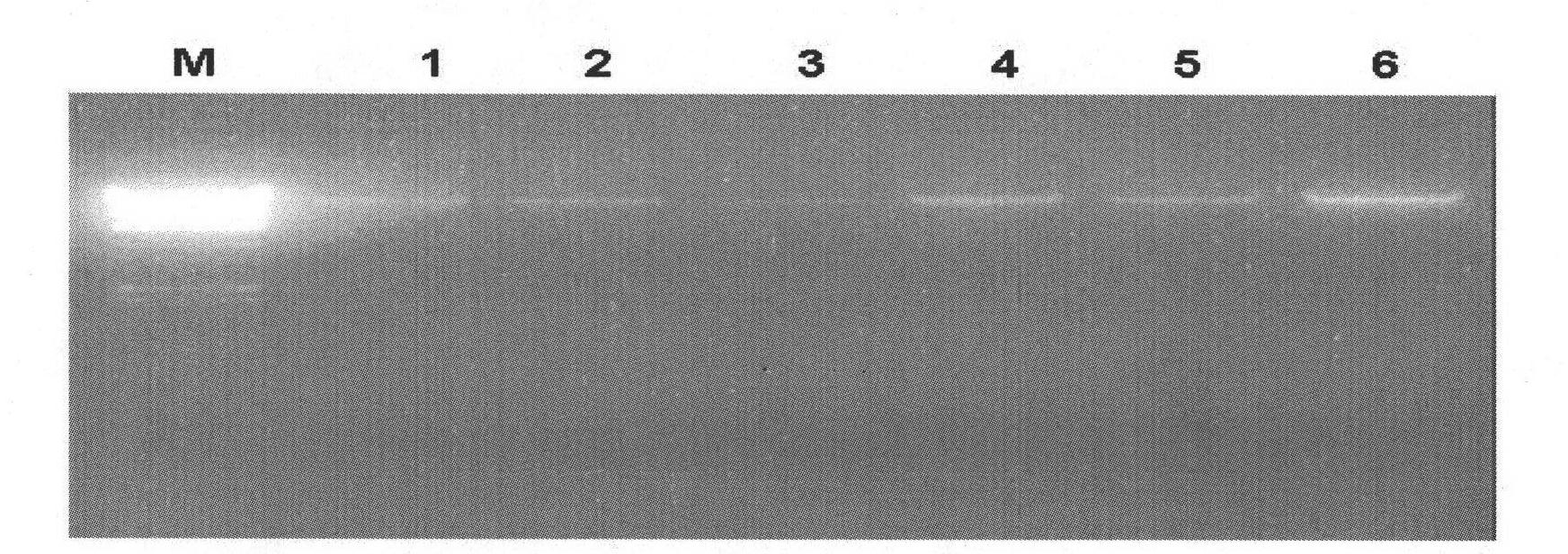
[0035] The Pichia pastoris (Candida boidinii) used in the present invention was purchased from China Industrial Microorganism Culture Collection Center, and the genomic DNA of Pichia pastoris was prepared by the CTAB method. Take 1.5mL of the bacterial liquid and centrifuge at 4000rpm for 2min at 4°C, discard the supernatant, and collect the bacterial cells. Add 400 μl 2×CTAB (Tris-HCl pH 7.5 100 mM, EDTA 20 mM, NaCl 1.4 M, CTAB 2%) to homogenate, incubate at 65° C. for 20 minutes, add 500 μl chloroform to mix, and centrifuge at room temperature at 13,000 rpm for 10 minutes. Transfer the supernatant, add 50 μl of 10% CTAB, add 650 μl of isopropanol and place at room temperature for 1 hour, centrifuge at 12,000 rpm at 4°C for 25 minutes, wash the precipitate once with 500 μl of 75% alcohol, dry it in vacuum, add 20 μl of TE containing RNase to dissolve, Incubate at 37°C for one hour. Get 2 μ l ge...
Embodiment 2
[0036] Example 2: Amplification and TA cloning of DAK gene:
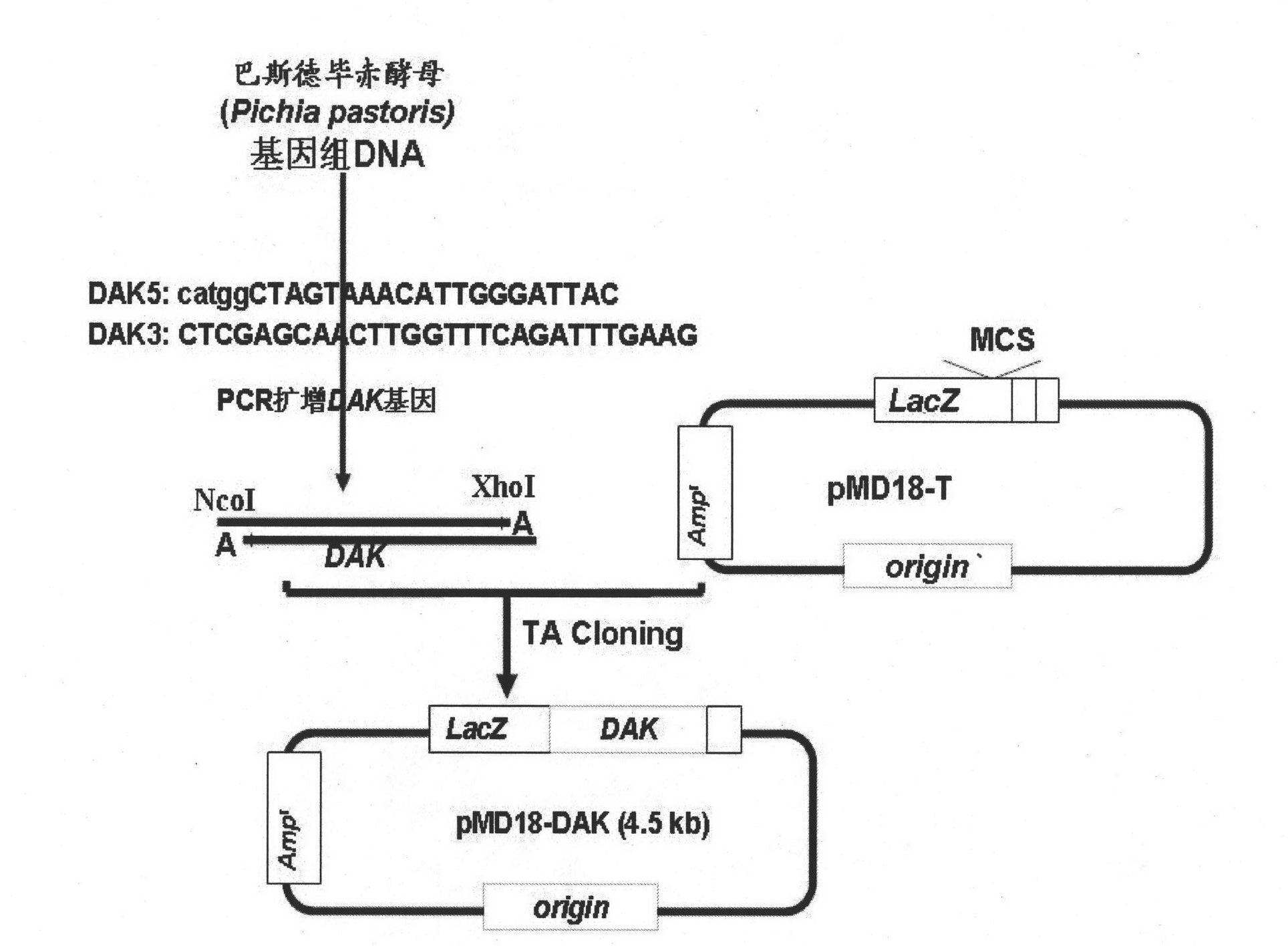
[0037] The amplification of DAK gene and the strategy of TA cloning are as follows: figure 2 As shown, first search the full-length gene sequence of DAK from GenBank (the GenBank accession number of DAK gene is AF019198), and design a pair of primers, the sequence is as follows:
[0038] DAK5: CATGGCTAGTAAACATTGGGATTAC
[0039] DAK3: CTCGAGCAACTTGGTTTCAGATTTGAAG
[0040] A C is added to the end of primer DAK5 at the 5' end to form an NcoI restriction site; an XhoI restriction site is added to the end of primer DAK3 at the 3' end.
[0041] Add 10 μg of Pichia pastoris genomic DNA as a template to the PCR reaction mixture, add 50 μg of specific primers DAK5 and DAK3, 1.8 μl of ldNTP (2.5 mM), 5 μl of Long Taq reaction buffer and 0.3 μl of long Taq (2.5 U / μl) polymerase (purchased from Tiangen Biochemical Technology), and double distilled water was added to make the final reaction volume 20 μl. Heat at 94°C for 3 ...
Embodiment 3
[0042] Embodiment 3: Construction of prokaryotic expression vector pET28a-DAK:
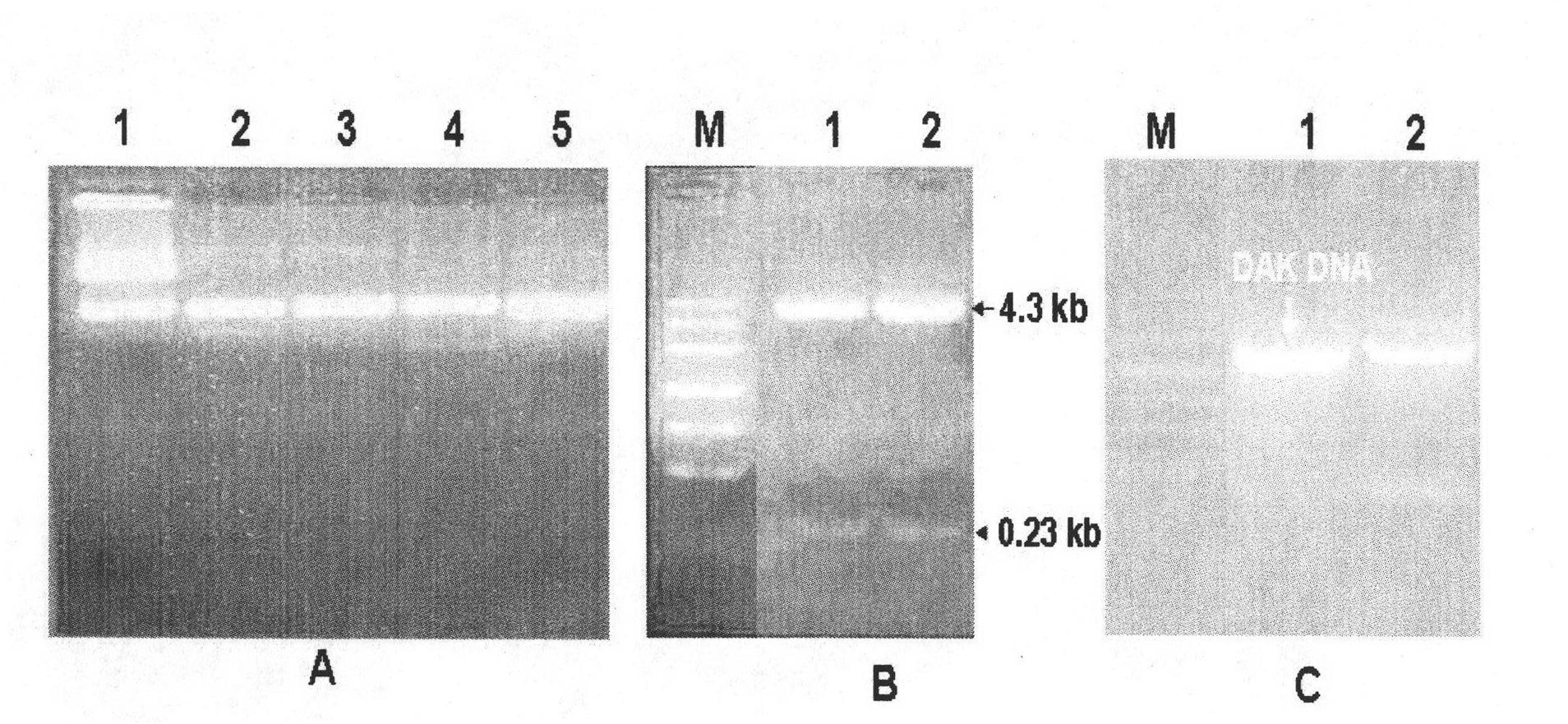
[0043] The construction strategy of pET28a-DAK is as follows Figure 4 As shown, the purified prokaryotic expression plasmid vectors pET28a (purchased from Novagen) and pMD18-DAK were cut with NcoI (Fermentas) and XhoI (Fermentas), and the cut vectors and inserts were separated by agarose gel electrophoresis. The vector fragment pET28a (5.4kb) generated after pET28a was cut and the DNA fragment (1.8kb) of the DAK gene generated by cutting pMD18-DAK were recovered from the gel, and then the pET28a vector was ligated with the ligase kit of TaKaRa Fragmentation and DNA fragmentation of the DAK gene to generate the prokaryotic expression vector pET28a-DAK. Conversion of high efficiency (10 8 ) Escherichia coli competent cells (DH5α, purchased from Tiangen Biochemical Technology), spread the transformed Escherichia coli on a plate added with kanamycin (Km, 50 μg / ml), cultivate overnight at 37°C, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com