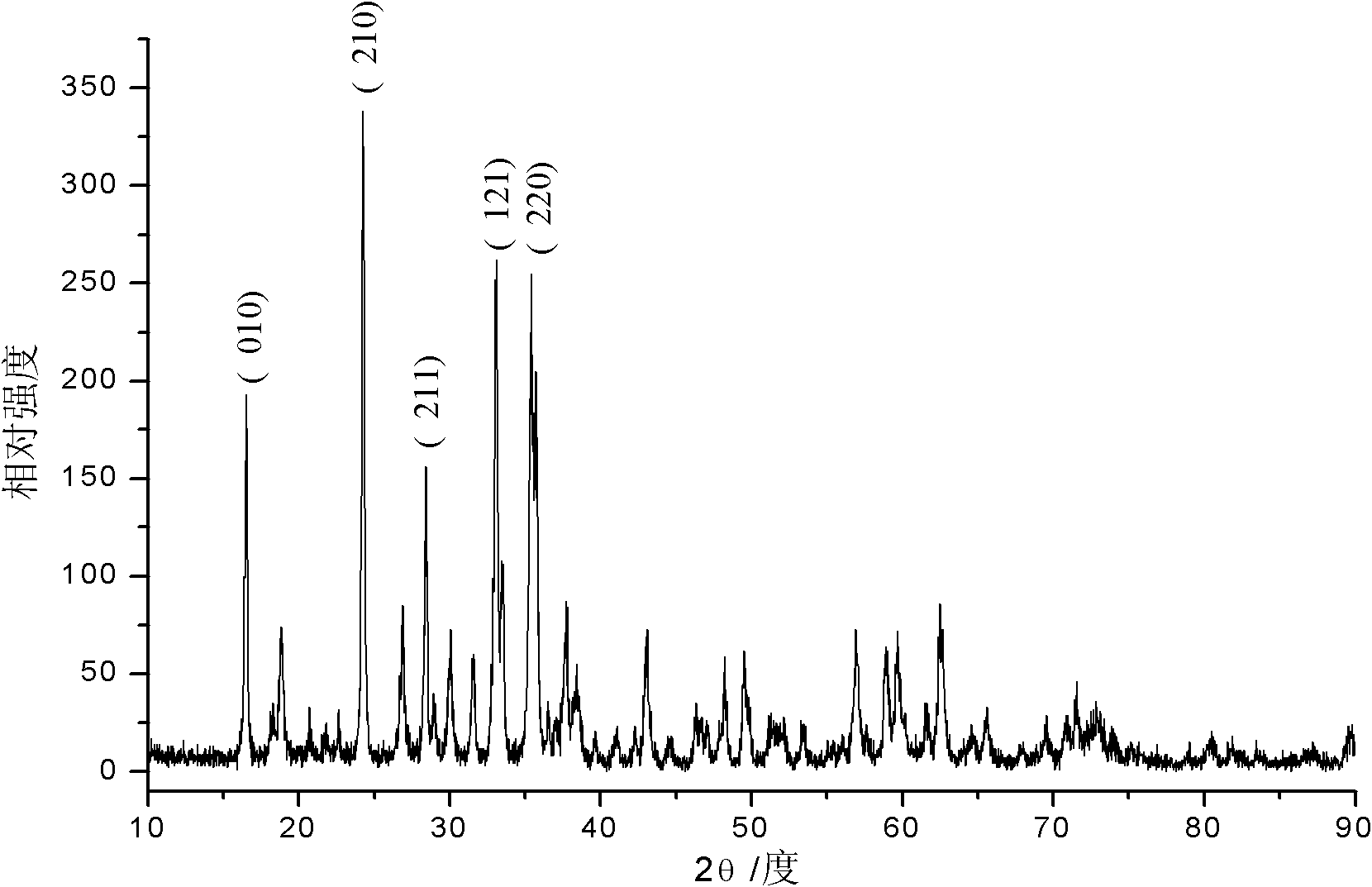
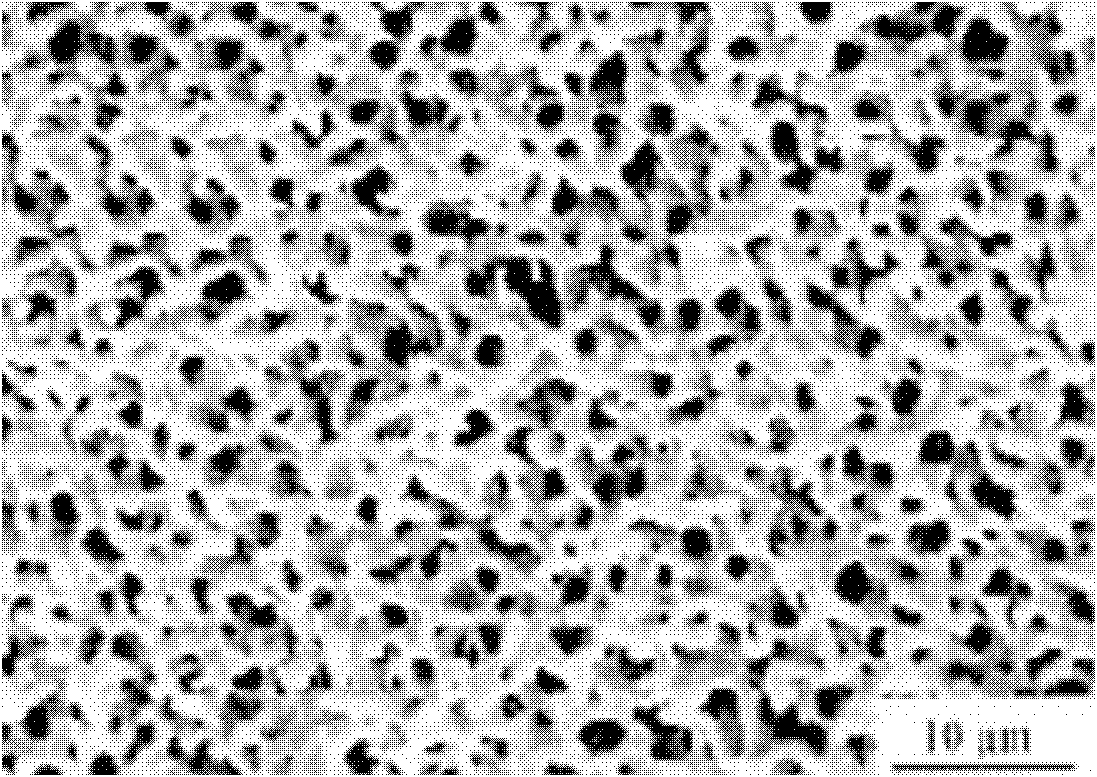
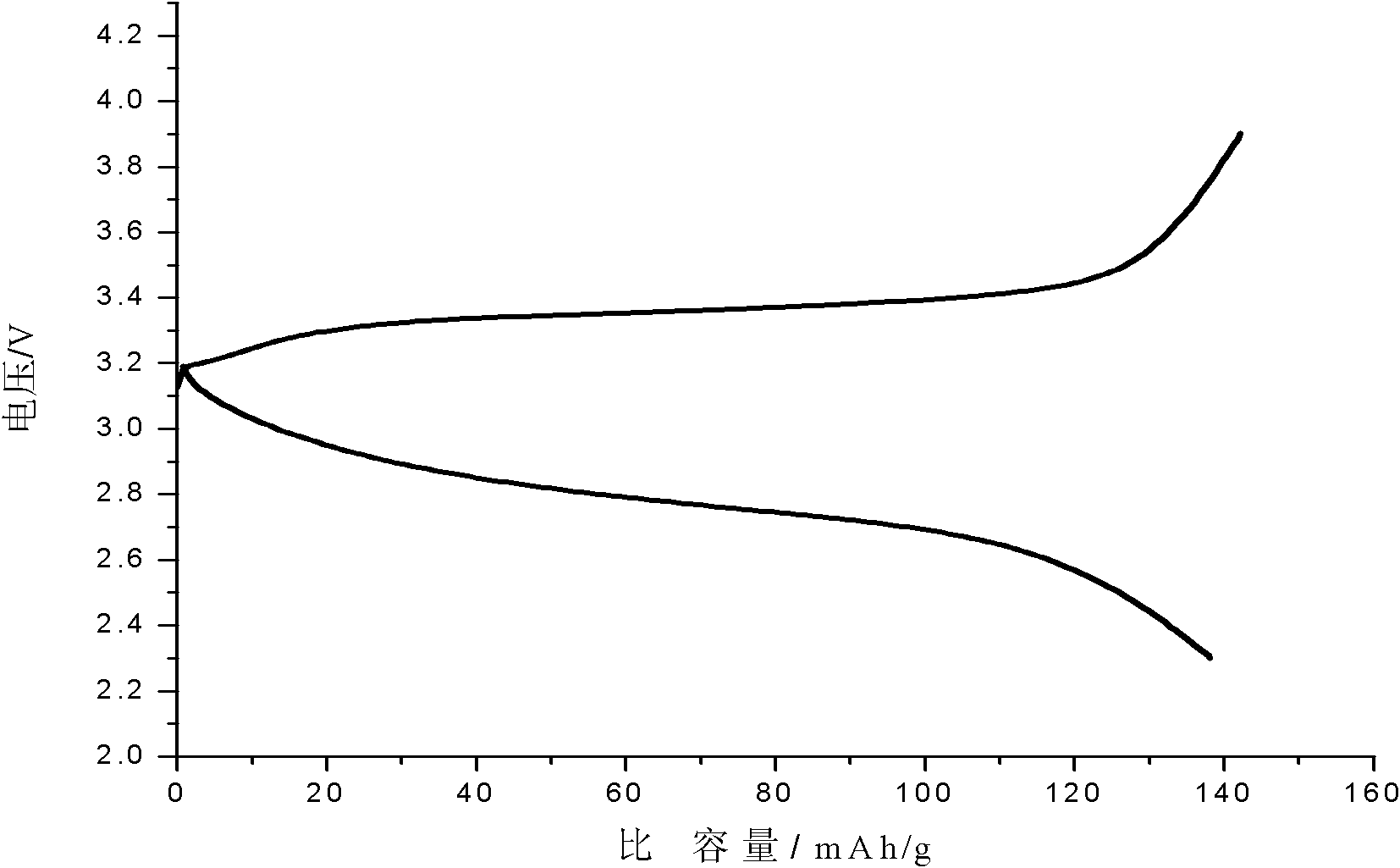
Porous carbon coated ferrous silicate lithium anode material and preparation method thereof
A technology of lithium ferrosilicate and cathode material, applied in battery electrodes, electrical components, circuits, etc., can solve the problem of affecting the charge and discharge current density and specific capacity of materials, limiting the application of lithium ferrosilicate, and affecting the electrochemical performance of products. and other problems, to achieve the effect of increasing the solid-liquid contact interface area, improving the electrochemical performance, and improving the specific capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]Lithium acetate, ferrous acetate, and ethyl orthosilicate are used as raw materials, and the corresponding substances are weighed according to the ratio of substances, so that the mass ratio of Li:Fe:Si is 2:1:1, and 1.640g of lithium acetate is accurately weighed, 1.8ml of tetraethyl orthosilicate and 0.399g of polyethylene glycol were dissolved in 13.3ml of ethanol so that the concentration of tetraethyl orthosilicate was 0.6mol / L. The obtained solution system was fully stirred for 10 min and mixed evenly. Accurately weigh 1.391g ferrous acetate, accurately weigh 0.039g ascorbic acid, add it to the above solution, stir to dissolve, and adjust the pH value to 7 with 0.3mol / L ammonia water as a catalyst. The mixed solution was transferred to a reaction kettle, and the reaction kettle was placed in a constant temperature oven to react at 120°C for 20 hours, and the obtained gel mixture was dried in a vacuum oven at 60°C to obtain a xerogel.
[0020] in N 2 Under the pro...
Embodiment 2
[0022] Lithium acetate, ferrous acetate, and ethyl orthosilicate are used as raw materials, and the corresponding substances are weighed according to the ratio of substances, so that the mass ratio of Li:Fe:Si is 2:1:1, and 1.640g of lithium acetate is accurately weighed, 1.8ml of tetraethyl orthosilicate and 0.129g of polyethylene glycol were dissolved in 10.0ml of ethanol so that the concentration of tetraethyl orthosilicate was 0.8mol / L. The obtained solution system was fully stirred for 10 min and mixed evenly. Accurately weigh 1.391g of ferrous acetate and 0.039g of ascorbic acid, add them to the above solution, stir and dissolve, and adjust the pH value to 6 with 0.2mol / L ammonia water as a catalyst. The mixed solution was transferred to a reaction kettle, and the reaction kettle was placed in a constant temperature oven at 140° C. for 17 hours. The obtained gel mixture was dried in a vacuum oven at 70° C. to obtain a xerogel.
[0023] in N 2 Under the protection of th...
Embodiment 3
[0025] Lithium acetate, ferrous acetate, and ethyl orthosilicate are used as raw materials, and the corresponding substances are weighed according to the ratio of substances, so that the mass ratio of Li:Fe:Si is 2:1:1, and 1.640g of lithium acetate is accurately weighed, 1.8ml of tetraethyl orthosilicate and 0.399g of polyethylene glycol were dissolved in 16.0ml of ethanol so that the concentration of tetraethyl orthosilicate was 0.53mol / L. The obtained solution system was fully stirred for 10 min and mixed evenly. Accurately weigh 1.391g of ferrous acetate, accurately weigh 0.039g of ascorbic acid, add it to the above solution, stir and dissolve, and adjust the pH value to 4 with 0.3mol / L ammonia water as a catalyst. The mixed solution was transferred to a reaction kettle, and the reaction kettle was placed in a constant temperature oven at 160° C. for 15 hours. The obtained gel mixture was dried in a vacuum oven at 80° C. to obtain a xerogel.
[0026] in N 2 Under the pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average pore diameter of large pores | aaaaa | aaaaa |
| Mesopore average pore size | aaaaa | aaaaa |
| Average pore diameter of large pores | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com