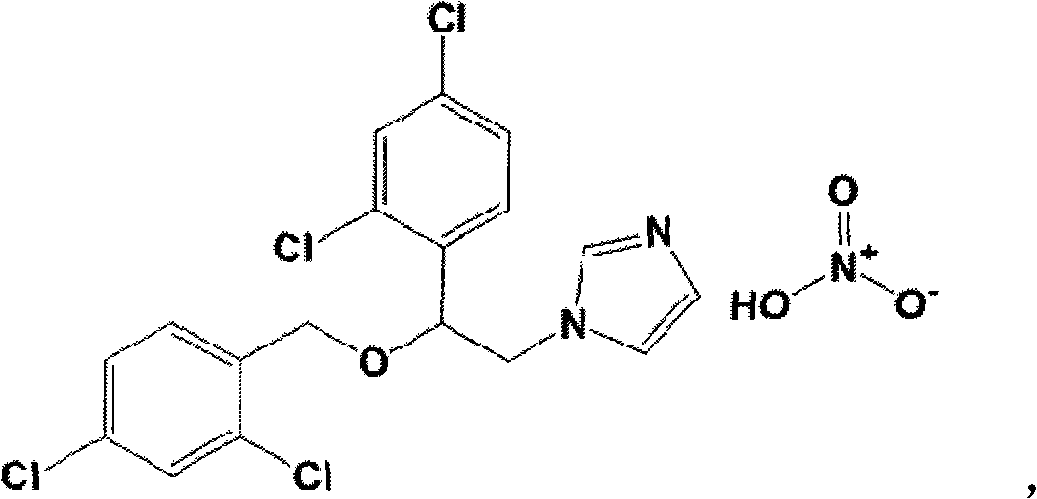
Method for producing miconazole nitrate on industrialized basis
A technology of miconazole nitrate and dichlorophenyl, which is applied in the field of raw material drug synthesis, can solve the problems of complicated operation of miconazole nitrate, difficult solvent recovery, and difficulty in obtaining raw materials, and achieves saving of raw materials, low cost, and easy availability of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for industrialized production of miconazole nitrate, the steps are as follows:
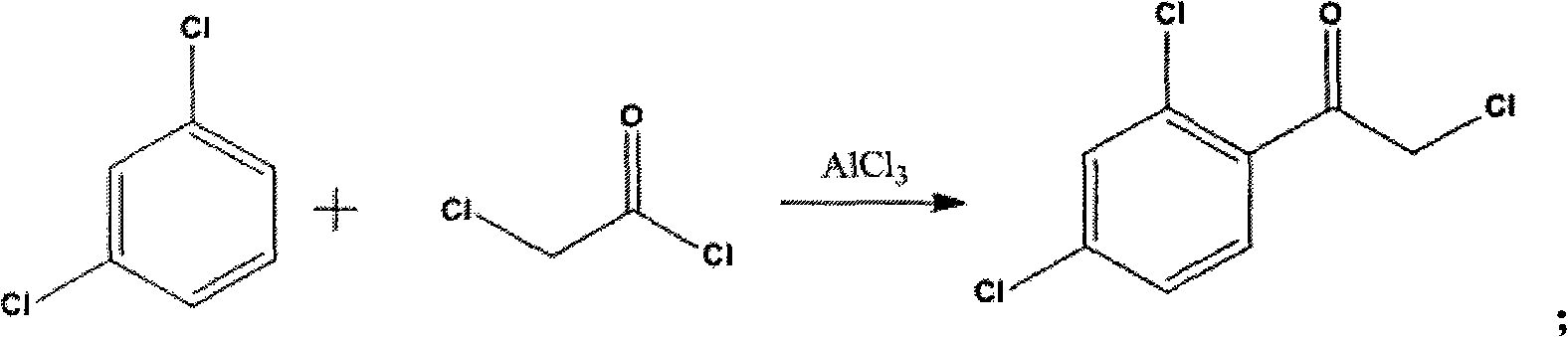
[0027] A, the preparation of intermediate 1
[0028] Mix 1 mol of chloroacetyl chloride with 1.1 mol of anhydrous AlCl 3 Mix well, then add 1.1mol m-dichlorobenzene dropwise, and react at 40°C for 5h after the dropwise addition is completed for 30-40min.
[0029] After the reaction was completed, the reaction solution was slowly added to 1.3 L of ice water, stirred, and filtered to obtain the crude intermediate 1. The crude product of intermediate 1 was recrystallized from n-hexane, and the melting point of intermediate 1 was measured at 52-54° C., and the yield was 92%.
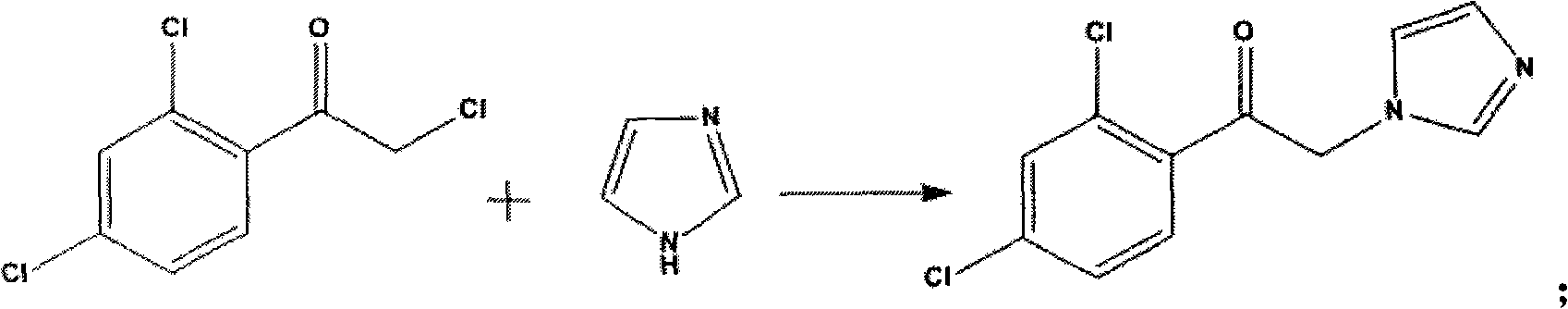
[0030] B, the preparation of intermediate 2
[0031] Suspend 3 mol of imidazole in 1 L of dichloromethane, add 1 mol of intermediate 1 obtained in step A and stir for 30 min, then heat to reflux for 5 h. After the reaction was completed, dichloromethane was distilled off at -0.06~-0.08MPa, the residue was add...
Embodiment 2
[0037] A method for industrialized production of miconazole nitrate, the steps are as follows:
[0038] A, the preparation of intermediate 1
[0039] Mix 1 mol of chloroacetyl chloride with 2 mol of anhydrous AlCl 3 Mix well, then add 2 mol of m-dichlorobenzene dropwise, after 30-40min dropwise, react at 40°C for 3h.
[0040] After the reaction was completed, the reaction solution was slowly added to 1.3 L of ice water, stirred, and filtered to obtain the crude intermediate 1. The crude product of intermediate 1 was recrystallized from n-hexane to obtain the melting point of intermediate 1: 52-54° C., and the yield was 91%.
[0041] B, the preparation of intermediate 2
[0042] Suspend 1 mol of imidazole in 1 L of dichloromethane, add 1 mol of intermediate 1 obtained in Step A and stir for 30 min, then heat to reflux for 5 h. After the reaction was completed, dichloromethane was distilled off at -0.06~-0.08MPa, and the residue was dissolved in 1.5L of water with vigorous s...
Embodiment 3
[0048] A method for industrialized production of miconazole nitrate, the steps are as follows:
[0049] A, the preparation of intermediate 1
[0050] Mix 1 mol of chloroacetyl chloride with 1.3 mol of anhydrous AlCl 3 Mix well, then add 1.6mol m-dichlorobenzene dropwise, and react at 40°C for 4h after the dropwise addition is completed for 30-40min.
[0051] After the reaction was completed, the reaction solution was slowly added to 1.3 L of ice water, stirred, and filtered to obtain the crude intermediate 1. The crude product of intermediate 1 was recrystallized from n-hexane to obtain the melting point of intermediate 1: 52-54° C., and the yield was 93%.
[0052] B, the preparation of intermediate 2
[0053] Suspend 2 mol of imidazole in 1 L of dichloromethane, add 1 mol of intermediate 1 obtained in step A and stir for 30 min, then heat to reflux for 5 h. After the reaction was completed, dichloromethane was distilled off at -0.06~-0.08MPa, and the residue was dissolved...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com