Peptide - cisplatin conjugate and preparation method and application thereof
A technology for the synthesis of conjugates and solid-phase peptides, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of human immune response, allergic symptoms, small molecular weight, etc., achieve low toxicity and side effects, and increase specificity. resistance, to avoid the effect of accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 polypeptide-cisplatin conjugate
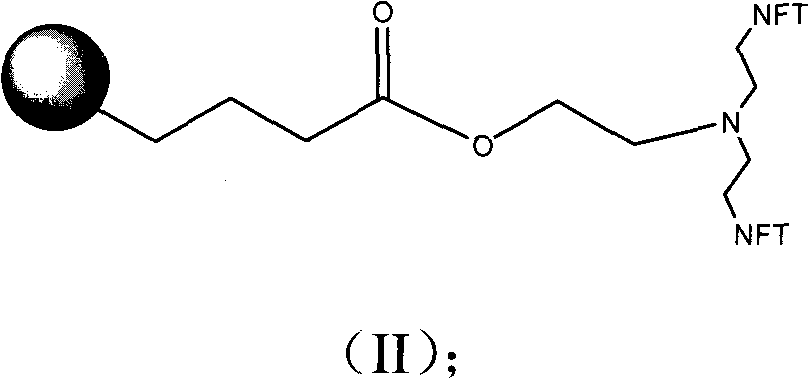
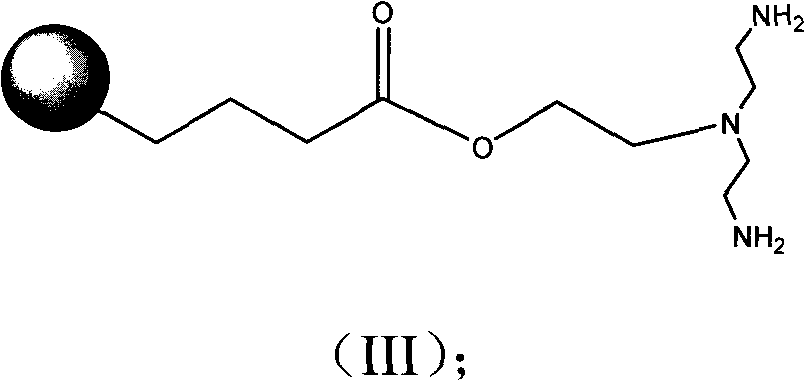
[0046] Add 10 ml of DMF, 25 mg (0.017 mol) of LLADTTHHRPWT, 3.5 mg (0.019 mmol) of 1,2-dibromoethane and 15 mg of anhydrous sodium carbonate into a 25 ml round bottom flask. Stir at room temperature for 24 hours. 10 mg (0.028 mol) of bis(2-phthalimide)ethylamine was added, and stirring was continued for 24 hours. 1 ml of 80% aqueous hydrazine solution was added, and stirring was continued for 3 days. Add 10 ml of 70% acetone solution and filter. The precipitate was washed with 70% acetone solution. The precipitate was dissolved in 3 ml of water, 4.5 mg (0.017 mmol) of platinum subchloride was added, and the solution was lyophilized to obtain the product.
Embodiment 2
[0047] Example 2 Anti-lung cancer pharmacodynamics study of polypeptide-cisplatin conjugates in vivo
[0048] 1 Materials and methods
[0049]1.1 cells
[0050] NCI-H1299 human lung cancer cell line was provided by the Shanghai Cell Institute of the Chinese Academy of Sciences.
[0051] 1.2 Experimental animals
[0052] SPF-grade healthy male Balb-c nude mice, 5-6 weeks old, weighing 16-20 g, were provided by Guangdong Medical Experimental Animal Center, certificate number: SCXK (Guangdong) 2008-0002. In the IVC system of the New Drug Screening and Pharmacodynamic Evaluation Center of the School of Pharmaceutical Sciences, Guangdong College of Pharmaceutical Sciences, the temperature is 25°C, and the humidity is 70-80%.
[0053] 1.3 Main Reagents and Drugs
[0054] High-glucose DMEM medium was purchased from Gibco; imported fetal bovine serum was purchased from Hyclone; test drug: H001, homemade injection; positive control drug: cisplatin, produced by Bayer, Germany, batch...
Embodiment 3
[0104] Example 3 Experimental Study on the Pharmacodynamics of Polypeptide-Cisplatin Conjugates Against Colon Cancer in Vitro
[0105] 1 Materials and methods
[0106] 1.1 Cell lines
[0107] The SW1116 human colon cancer cell line was provided by the Shanghai Cell Institute of the Chinese Academy of Sciences.
[0108] 1.3 Main Reagents and Drugs
[0109] High-glucose DMEM medium was purchased from Gibco; imported fetal bovine serum was purchased from Hyclone; test drug: H001, homemade injection; positive control drug: cisplatin, produced by Bayer, Germany, batch number: BXF 23E3.
[0110] 1.4 Cell culture and passage
[0111] Human colon cancer cells SW1116 were cultured in high-glucose DMEM medium containing 10% fetal bovine serum at 37°C and 5% CO 2 Cultured in a constant temperature incubator, the cells grow as a single layer of adherent cells. When the adherent cells reach 80%-90% confluence, they are digested and passaged with 0.25% trypsin containing 0.01% EDTA.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com