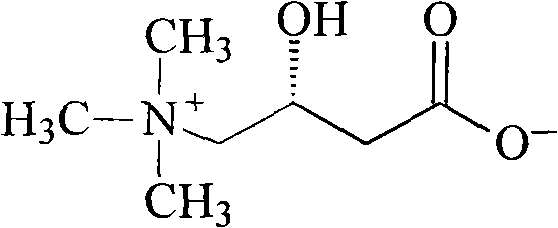
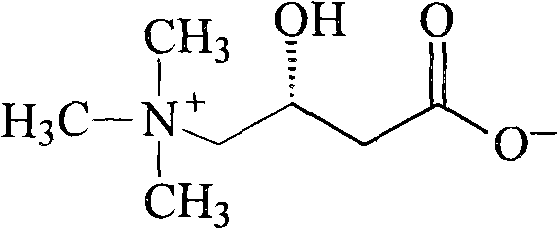
Levocarnitine compound and new preparation method thereof
A synthesis method and catalyst technology, applied in the field of levocarnitine compound and its new method, can solve the problems of cumbersome steps, low yield, expensive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
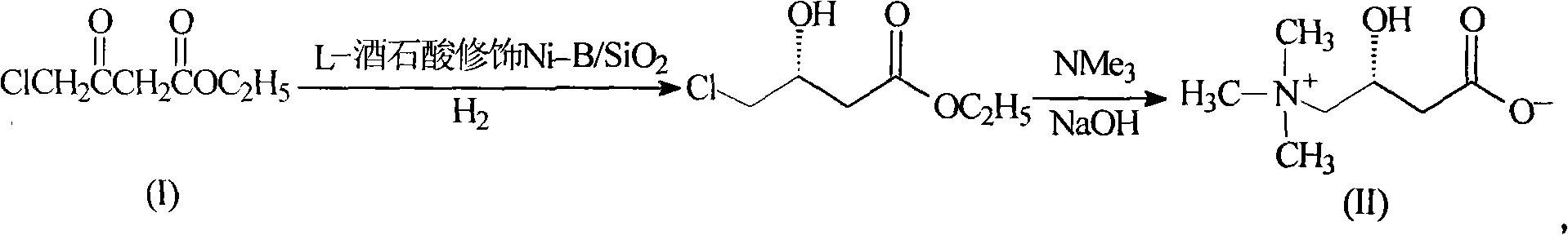
[0037] The synthesis of embodiment 1 (R)-4-chloro-3-hydroxyl-butyric acid ethyl ester
[0038] Dissolve 165g (1mol) of ethyl 4-chloroacetoacetate in 300ml of ethanol, add it to the hydrogenation reactor, and then add 10g of L-tartaric acid to modify Ni-B / SiO 2 Catalyst, close the reactor, replace the air in the reactor with nitrogen, then replace the nitrogen with hydrogen, keep the pressure of the reactor at 10kg, then increase the temperature and control it at 60°C, react for 5 hours, leave it to cool, and concentrate the reaction mixture under reduced pressure , the concentrate was distilled under high vacuum, and the fraction at 70° C. (about 0.5 mmHg) was collected to obtain 155 g of (R)-4-chloro-3-hydroxyl-butyric acid ethyl ester, a colorless transparent liquid. The yield: 93%, and the optical purity was 98%.
Embodiment 2
[0039] The synthesis of embodiment 2 levocarnitine
[0040]Dissolve 24g (0.6mol) of sodium hydroxide in 600ml of 10% (1mol) trimethylamine aqueous solution, and slowly add dropwise to 84g (0.5mol) (R)-4-chloro-3-hydroxyl at 0-5°C - Dissolve ethyl butyrate in a solution formed by 1000ml of chloroform, control the drop rate at 4-6ml / min, continue to stir and react at 0-5°C for 15 hours, then gradually rise to room temperature, react for 24 hours, and remove by layers For the organic phase, concentrate the aqueous phase, stir and add Ambertlite IR-120 resin, absorb for 30 minutes, wash the resin three times with depurified water, and then wash the product three times with 10% ammonia water, combine the ammonia solution, concentrate, and obtain a white solid levocarni Tin product 68.1g, yield 84.1%, [α] 20 =-29.5° (c=1, H 2 O).
Embodiment 3
[0041] The synthesis of embodiment 3 (R)-4-chloro-3-hydroxyl-butyric acid ethyl ester
[0042] Dissolve 165g (1mol) of ethyl 4-chloroacetoacetate in 300ml of ethanol, add it to the hydrogenation reactor, and then add 10g of L-tartaric acid to modify Ni-B / SiO 2 Catalyst, close the reactor, replace the air in the reactor with nitrogen, then replace the nitrogen with hydrogen, keep the pressure of the reactor at 12kg, then increase the temperature and control the reaction at 50°C, react for 6 hours, leave it to cool, and concentrate the reaction mixture under reduced pressure , the concentrate was distilled under high vacuum, and the fraction at 70°C (about 0.5mmHg) was collected to obtain 157.5g of (R)-4-chloro-3-hydroxy-butyric acid ethyl ester, a colorless transparent liquid, yield: 94.5%, optical purity 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com