Humanized monoclonal antibody IgG Fab fragment of dermatophagoides farinae 2 allergoid specificity as well as preparation method and application thereof
An allergen-like and dust mite technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, anti-animal/human immunoglobulin, etc., can solve the problems of unclear specific mechanism and achieve the reduction of allergic asthma Symptoms, effects of prolonging half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Construction of Antibody Library
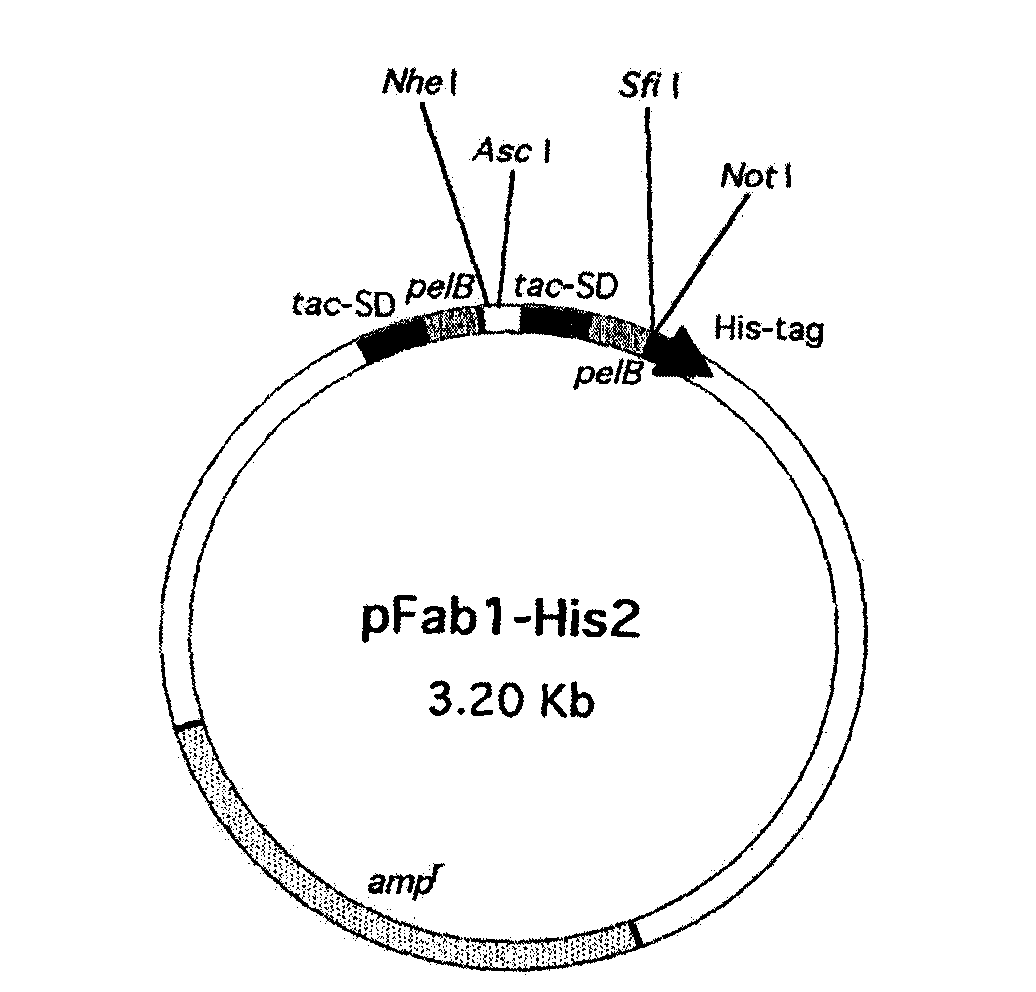
[0041] Select 300 informed healthy people (no history of allergic diseases and no allergen immunization) who have not suffered from infectious diseases such as colds in the past 2 months. , Sweden) to separate lymphocytes, and total RNA was extracted with a kit (QIAGEN GmbH, Hilden, Germany). The total RNA was reverse-transcribed into cDNA using the Gene-Amp RNA PCR kit (Perkin-Elmer Cetus, Norwalk, Conn) with Oligo(dT)16, and the upstream and downstream primers (Invitrogen ) (Table 1) for PCR amplification of immunoglobulin γ, κ, λ chain genes. After the PCR product was purified by a kit (QIAGEN GmbH, Hilden, Germany), the κ chain and λ chain products were double-digested with AscI and NheI (NEW ENGLAND BioLabs), respectively. The digested κ chain and λ chain products were combined with human immunoglobulin Fab expression vector pFab-His2( figure 1 ) connection, followed by electrotransformation into Escherichia coli JM1...
Embodiment 3
[0048] Example 3. Screening of positive clones specific to dust mite type 2 allergen (Der f2)
[0049] Take 9×10 8 Transform 100 μl JM109 Escherichia coli with DNA 10ng of antibody library independent of clonal titer, spread the bacterial solution on Luria broth (10g sodii chloridum, 10g tryptone, 5g yeast extract / L, PH 7) plate (containing 50 μg ampicillin / ml), cultured at 37°C for 7 hours, to be cloned (about 5×10 3 Clones / 90mm diameter plate) when the diameter is about 0.1-0.3mm, cover the plate with a nitrocellulose membrane (Armacia / Pharmacia) with a diameter of 82mm. On the LB plate, induce expression at 30°C for 6 hours, and then use lysozyme, DNase and bovine serum albumin (100mM Tris-HCl [pH 7], 150mM NaCl, 5mM MgCl 2 , 1.5% BSA, 1mg of DNase, 40mg lysozyme / ml) to lyse the membrane. After washing to remove residual bacterial debris on the membrane, block with bovine serum albumin (BSA), react with 250 μg of purified recombinant protein, and then react with positiv...
Embodiment 4
[0052] Example 4. Characteristic Analysis of Fab Fragments of Positive Clones
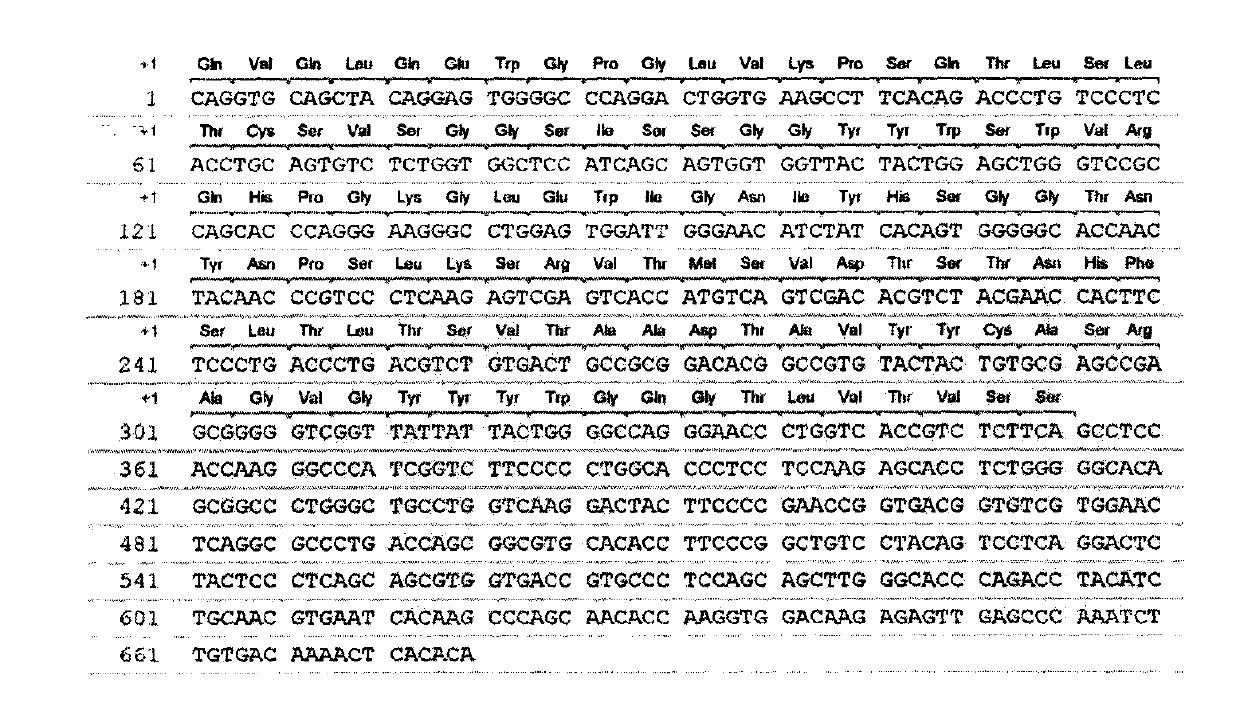
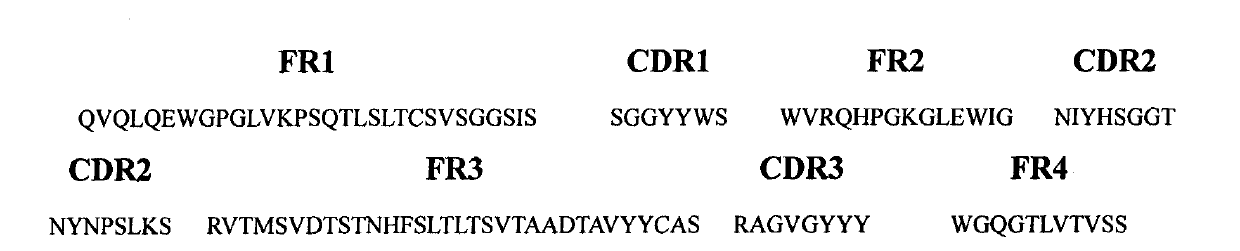
[0053] Take the plasmid of the positive clone AM1L-Hsh, digest it with AscI-NdeI and SfiI-NotI restriction endonucleases, respectively, to obtain the light chain and heavy chain genes, and then combine them with the modified sequencing vectors CV-2 and CV-1 Ligation, transformation of Escherichia coli JM109, extraction of plasmid DNA containing light chain or heavy chain genes, respectively, with M13Reverse primer (5'-GGATAACAATTTCACACAGG-3'), sequenced by Invitrogen. And calculate the amino acid sequence with VectorNTI 10 software ( figure 2 , 4), homology analysis (table 2) was carried out with IgBlast, and according to Kabat system, the CDR and FR of the light chain variable region and the heavy chain variable region of AM1L-Hsh were divided ( image 3 , 5)
[0054] Since the Fab antibody contains 6 His-Tags and the heavy chain is linked to His, His-binding resin (Novagen, Madison, Wis.) was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com