Doxorubicin hydrochloride liposome injection and preparation technology thereof
A technology for doxorubicin hydrochloride and injections, which is applied in the field of doxorubicin hydrochloride liposome injections, can solve the problems of long time required for freeze-drying, slow sublimation speed, high toxicity, etc., and achieve good uniformity and short time , The effect of less material exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of Doxorubicin Hydrochloride Liposomal Injection
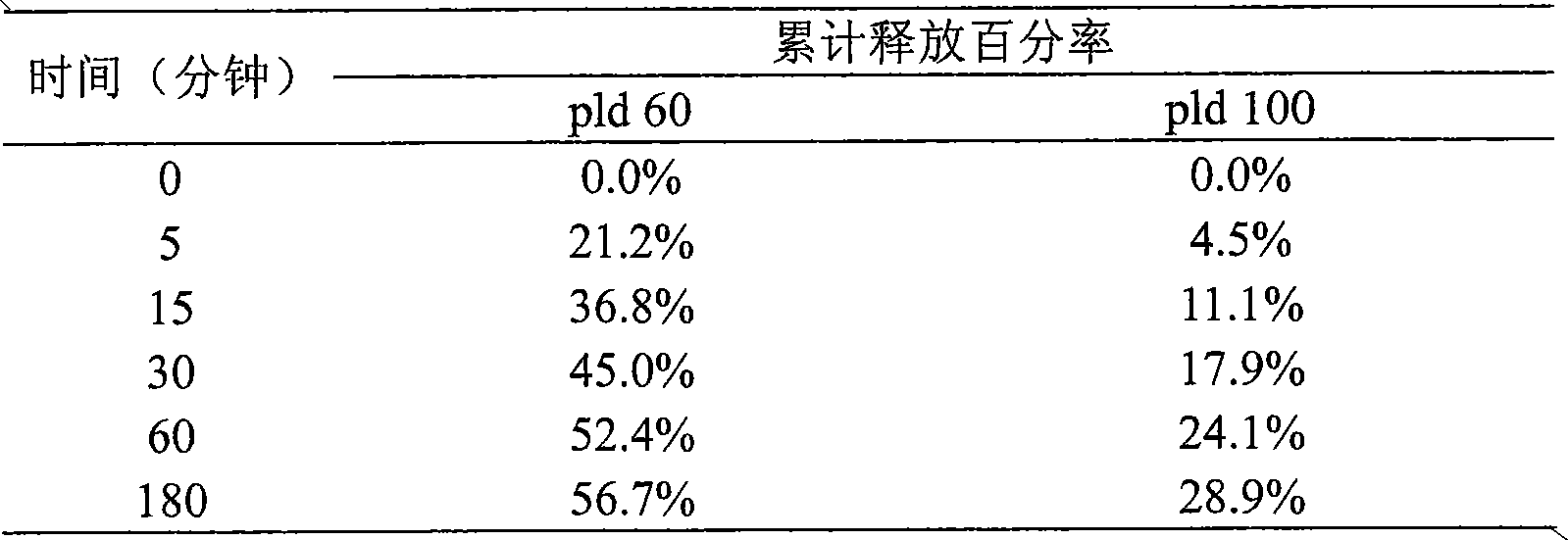
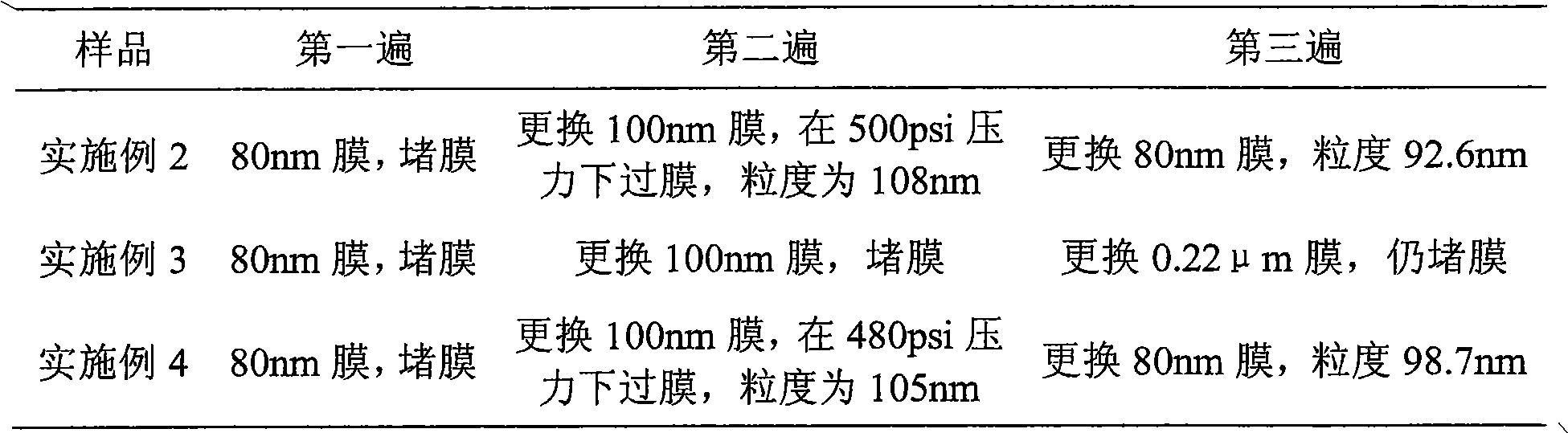
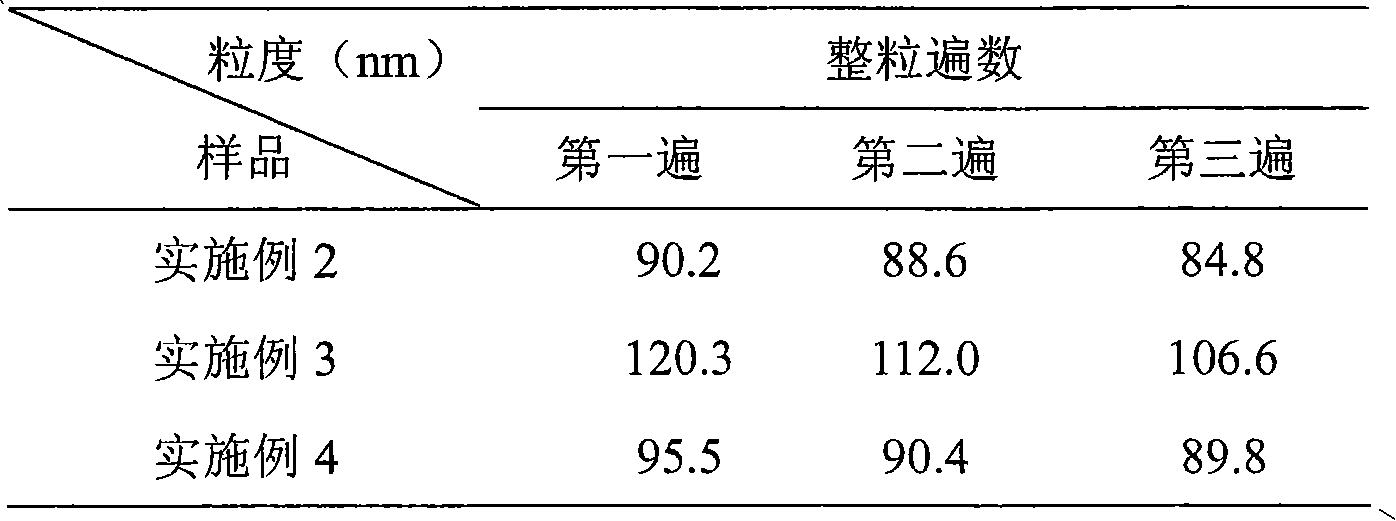
[0052] Mix HSPC (hydrogenated soybean lecithin), Chol (cholesterol) and DSPE-PEG2000 (methoxy PEG2000-distearate phosphatidylethanolamine) in a weight ratio of (3:1:1), dissolve in ethanol / tert-butyl In alcohol (volume ratio is 5:95), lyophilize and remove organic solvent in lyophilizer, form loose lipid phase mixture; Prepare the ammonium sulfate solution of 300mM, add in the lipid phase after lyophilization, at 60 ℃ Incubate and oscillate in a water bath for 30 minutes for hydration to obtain uneven blank liposomes. Whole the blank liposomes in a microfluidizer. After the obtained sample was diluted 200 times with 0.9% NaCl solution, the particle size was measured by NanoZS, and the average particle size of the particles was 100 nm. Using column chromatography, the outer phase was replaced with 250 mM sucrose and 50 mM glycine to form an ammonium sulfate gradient inside and outside the phosphol...
Embodiment 2
[0061] Example 2 Lipid phase mixing by lyophilization
[0062] HSPC, Chol and DSPE-PEG2000 were mixed according to the weight ratio of (3:1:1), dissolved in ethanol / tert-butanol (10:90 in volume ratio); lyophilized in a lyophilizer to remove the organic solvent to form Loose lipid phase mixture.
Embodiment 3
[0063] Embodiment 3 thin film dispersion method carries out lipid phase mixing
[0064] HSPC, Chol and DSPE-PEG2000 were mixed according to the weight ratio (3:1:1), dissolved in chloroform, and the organic solvent was evaporated under reduced pressure in a rotary evaporator to obtain a lipid dry film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com