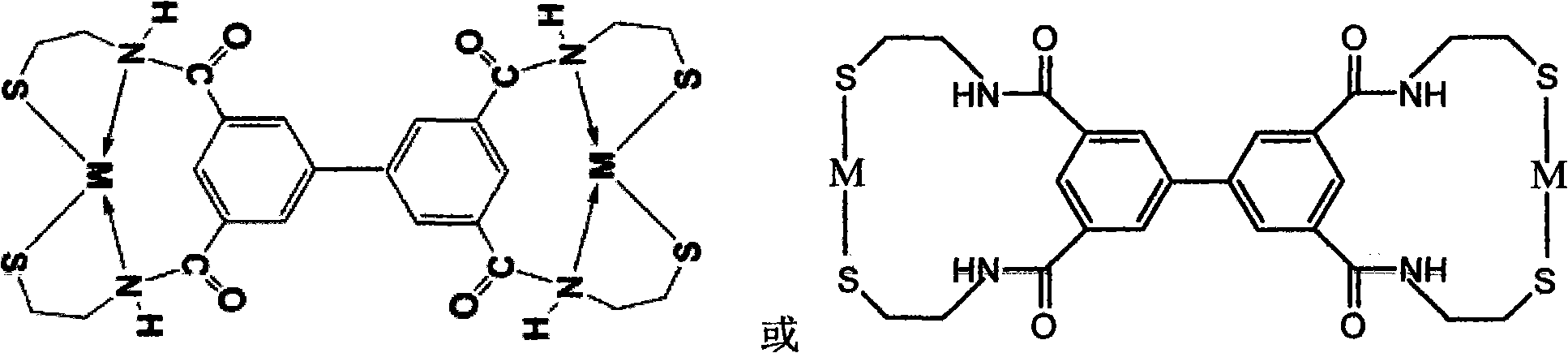
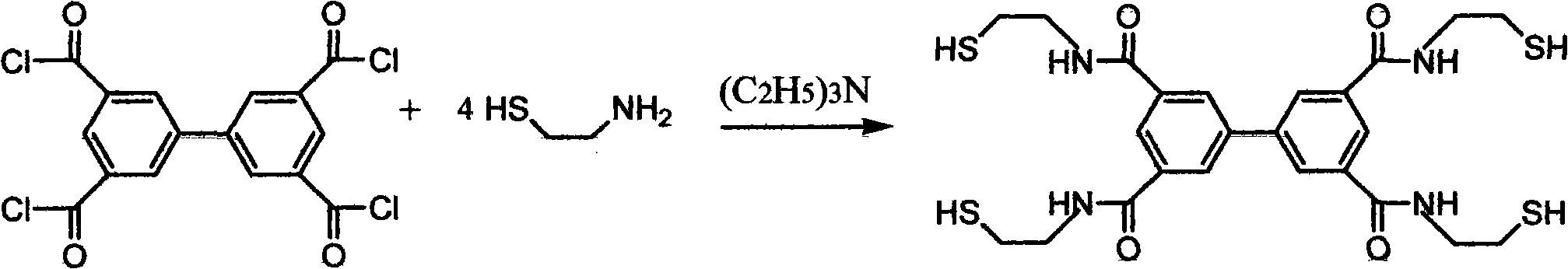Sulfide heavy metal chelating trapping agent and preparation method thereof
A technology for heavy metals and collectors, which is applied in the preparation of mercaptans, chemical instruments and methods, organic chemistry, etc., can solve the problems of difficult to ensure that the heavy metal wastewater fully meets the standard discharge, the sludge water content is large, and the precipitation is incomplete. The effect of secondary pollution, simple preparation process and shortening of settling time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Dissolve 550 grams of mercaptoethylamine hydrochloride in 3L of chloroform, add 900 grams of triethylamine as an acid-binding agent, and dissolve 400 grams of 3,3',5,5'-biphenyl tetracarbonyl chloride in 2L of chloroform, and control the stirring rate 30-100r.min -1 (The best is 50r.min -1 ), the above two solutions were slowly mixed under stirring, under the protection of nitrogen, the condensation reaction was carried out in an ice bath for 3 to 8 hours, then filtered, extracted with water and chloroform respectively, and the excess solvent was distilled out with a rotary evaporator, A white precipitate was obtained, which was the target product, a sulfide heavy metal chelating collector, and the reaction yield was 87%.
Embodiment 2
[0026] The circuit board wastewater from a company in Suzhou was selected, and the measured copper ion concentration was 206 mg.L -1 , pH 5.1. Add 950mg.L -1 The sulfide heavy metal chelating collector of embodiment 1, stirring speed is 30r.min -1 , after reacting and stirring for 5min, leave it to stand and filter, get the supernatant, and measure its copper ion concentration with an atomic absorption spectrophotometer (GBC company in Australia) to be 0.041mg.L respectively -1 , the concentration of copper ions is far less than the special discharge limit of 0.30 mg.L for water pollutants in the national discharge standard for electroplating pollutants GB21900-2008 -1 .
Embodiment 3
[0028] The electroplating wastewater from a company in Danyang, Jiangsu Province was selected, and the Zn 2+ The concentration is 5.52mg / L, Ni 2+ The concentration is 4.37mg / L. Add 60mg.L -1 The sodium salt of the sulfide heavy metal trapping agent of embodiment 1, stirring speed is 30r.min -1 , the reaction was stirred for 5 min and then filtered, and the supernatant was taken to measure Ni 2+ and Zn 2+ Concentrations are 0.044mg.L -1 , 0.097mg.L -1 , far less than the special discharge limit of 0.10 mg.L for water pollutants in the national discharge standard for electroplating pollutants GB21900-2008 -1 and 1.0mg.L -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com