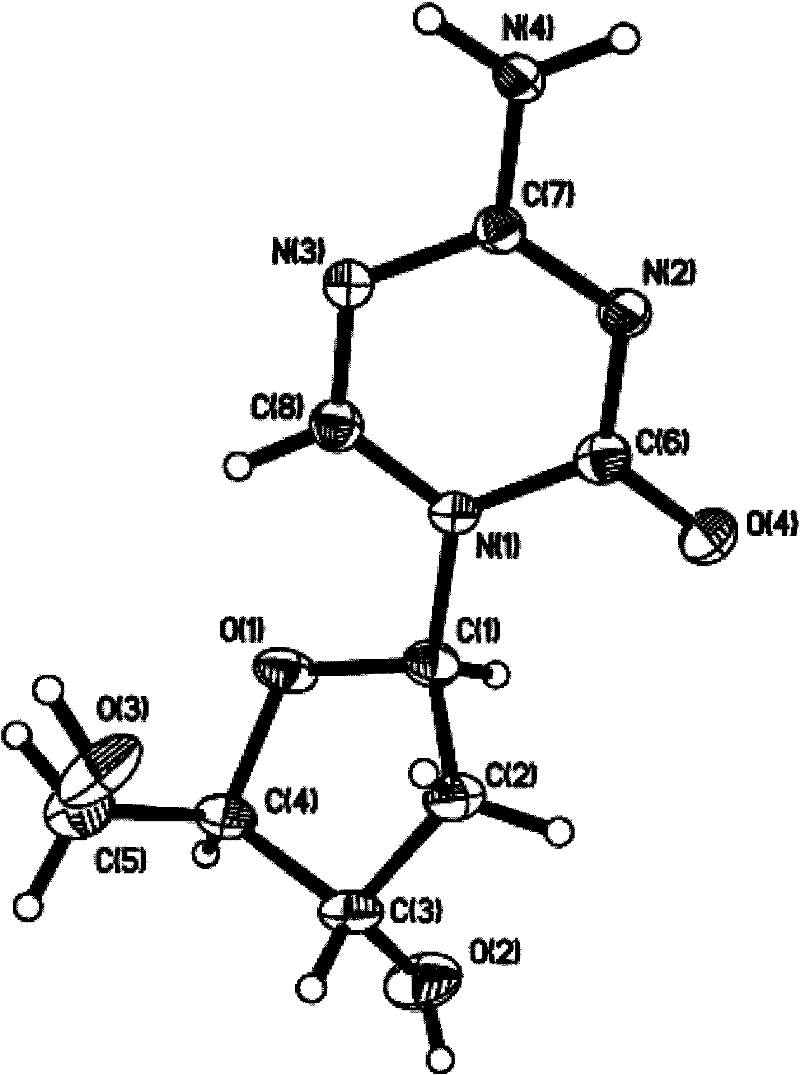
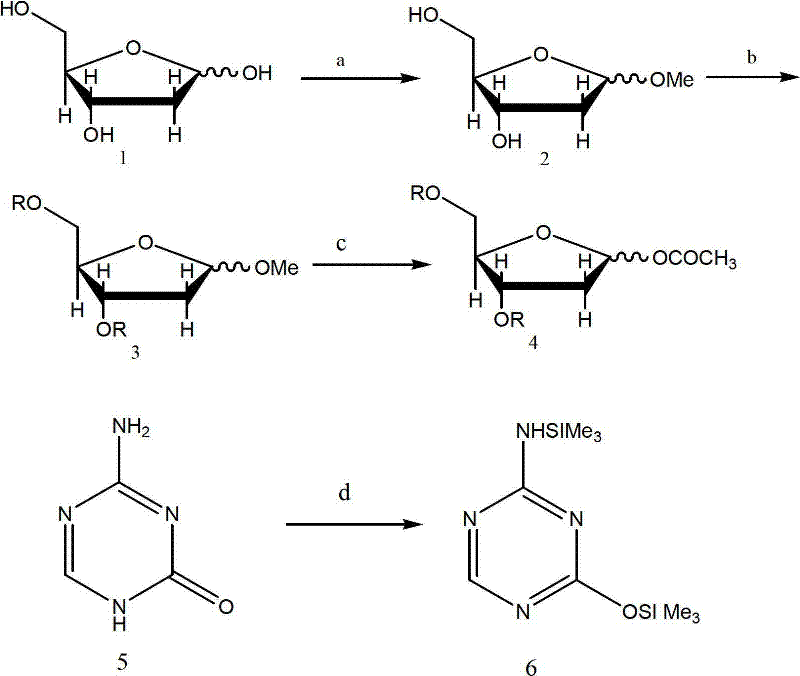
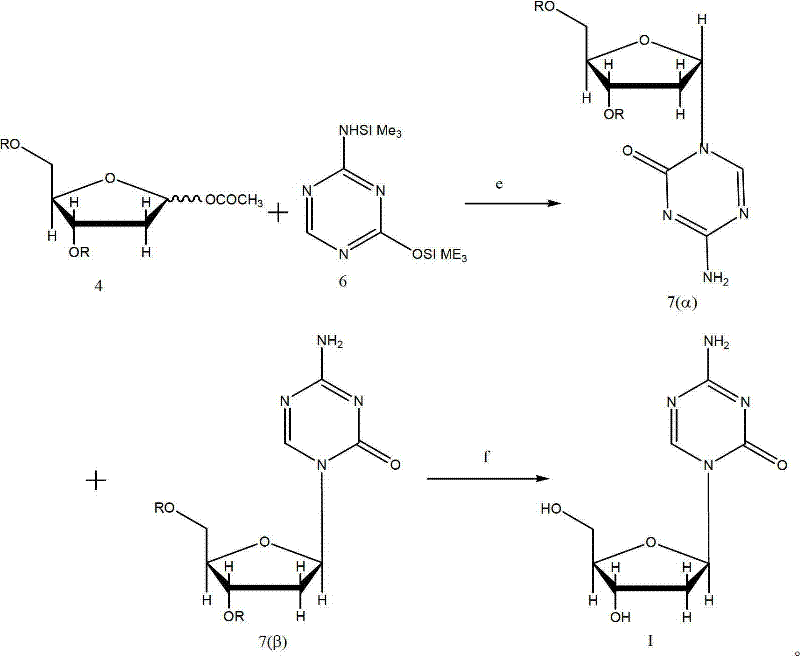
Preparation, separation and purification method of Decitabine
A separation and purification, decitabine technology, applied in the field of decitabine preparation and separation and purification, can solve the difficulty of isomer separation, product deprotection is difficult to carry out, does not provide isomer separation, purification method, etc. problems, to achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of embodiment 1 intermediate (2)
[0030] Add 500g of 2-deoxy-D-ribose and 3000ml of anhydrous methanol into a 5000ml three-necked flask, and stir at 25°C until the solid is completely dissolved. Stir for 2 hours after the addition is complete, and TLC detects that after the reaction is complete, add 130g of solid Ag 2 CO 3 , continue stirring for 3 hours, suction filtration, the filter cake is washed several times with 200ml of anhydrous methanol, the filtrates are combined, and then the solvent is evaporated under reduced pressure to dryness to obtain 550g of oily intermediate (2), and the yield is 95%.
Embodiment 2
[0031] The synthesis of embodiment 2 intermediate (2)
[0032]Add 500g of 2-deoxy-D-ribose and 3500ml of anhydrous methanol into a 5000ml three-necked flask, stir at 40°C until the solid is completely dissolved, the system is a light yellow transparent solution, add 1000ml of 1.2% HCI-methanol solution dropwise through a constant pressure funnel , stirred for 50 minutes after the dropwise addition, and detected by TLC. After the reaction was completed, 135g of solid Ag was added 2 CO 3 , continue stirring for 2 hours, suction filtration, the filter cake is washed several times with 200ml of anhydrous methanol, the filtrates are combined, and then the solvent is evaporated under reduced pressure to dryness to obtain 540g of oily intermediate (2), with a yield of 93.5%.
Embodiment 3
[0033] The synthesis of embodiment 3 intermediate (3)
[0034] In a 50000ml three-neck flask equipped with a drying tube, add 296.3g (2mol) of self-made intermediate (2), 2L anhydrous pyridine, control the internal temperature of the reaction system between -10 and 0°C with a low-temperature bath, and dropwise add 740g ( 2.4mol) p-methoxybenzoyl chloride, after the dropwise addition, place the reaction at room temperature for reaction, TLC detection, after the reaction is complete, add about 10L of ice water, 3L of chloroform and 1L of 10% HCI solution under rapid stirring, and stir evenly , add a 5000ml separatory funnel, separate the layers, wash the aqueous layer with 500mlx2 chloroform, combine the organic phases, anhydrous Na 2 SO 4 After drying, the solvent was evaporated to dryness under reduced pressure. 768 g of oily liquid was obtained as intermediate (3), and the yield was 93.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com