Preparation method of N-substituted 3-aminomethyl pyrrolidine
A technology of aminomethylpyrrolidine and sulfonyl group is applied in the field of preparation of pharmaceutical intermediates and can solve the problems of limited industrial application, danger and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
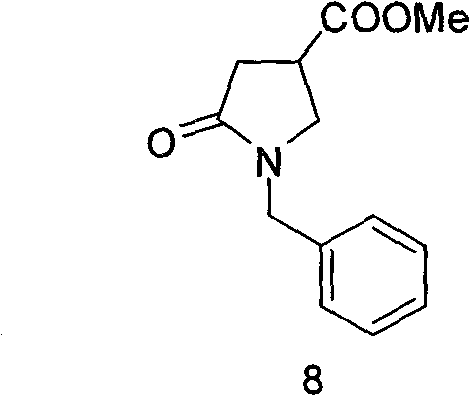
[0021] Preparation of methyl-1-benzyl-5-carbonylpyrrolidine-3-carboxylate (8)
[0022]
[0023] Add dimethyl itaconate (158.15 g, 1 mol) and benzylamine (107.15 g, 1 mol) into a 1 L three-neck round bottom flask, heat the oil bath to 90° and stir the reaction. The reaction was stopped after the completion of the reaction monitored by TLC. Cool to room temperature, spin dry, add 500mL of dichloromethane, 100mL of dilute hydrochloric acid, shake and separate the layers, separate the organic layer, add saturated sodium bicarbonate solution, shake and separate the layers, separate the organic layer, wash with saturated sodium chloride solution once, Dry over anhydrous sodium sulfate, spin dry, and recrystallize with ethyl acetate and petroleum ether to obtain 200 g of product with a yield of 86%.
Embodiment 2
[0025] Preparation of (1-benzylpyrrolidin-3-yl)methanol (9)
[0026]
[0027] In a 1L three-neck round-bottomed flask, add lithium aluminum tetrahydride (40g, 1.07mol), add 1.2L tetrahydrofuran under ice bath, add human compound (8) (100g, 0.43mol), remove the ice bath, and heat up in the oil bath To 66 ° of reaction, TLC monitors the end of the reaction. Cool to room temperature, add 10% NaOH solution, filter with suction, spin dry the filtrate, add 200 mL of water, adjust the pH to 9-10 with solid sodium carbonate, extract the product with dichloromethane, wash the organic layer with saturated sodium chloride, and dry with anhydrous sodium sulfate , spin-dried to obtain 80g product, yield 97%.
Embodiment 3
[0029] Preparation of (1-benzylpyrrolidin-3-yl)methyl methanesulfonate (10)
[0030]
[0031] In a 1L single-necked flask, add compound (9) (75g, 0.39mol), triethylamine (47mL, 59mol), methanesulfonyl chloride (36mL, 0.47mol), 375mL tetrahydrofuran, react at room temperature, and monitor the completion of the reaction by TLC. Add saturated sodium bicarbonate solution to make the pH of the aqueous phase 7-8, extract the product with ethyl acetate, wash the organic layer with water, wash the organic layer with saturated sodium chloride, dry with anhydrous sodium sulfate, and spin dry to obtain 90g of product with a yield of 86 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com