Method for preparing quaternary ammonium salt containing adamantine alkyl
A technology of adamantane trimethylammonium halide salt and adamantyl, which is applied in the field of preparation of quaternary ammonium salts with adamantyl, and can solve the problems of incomplete reaction of raw materials, impossibility of purification, impossibility of improving purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
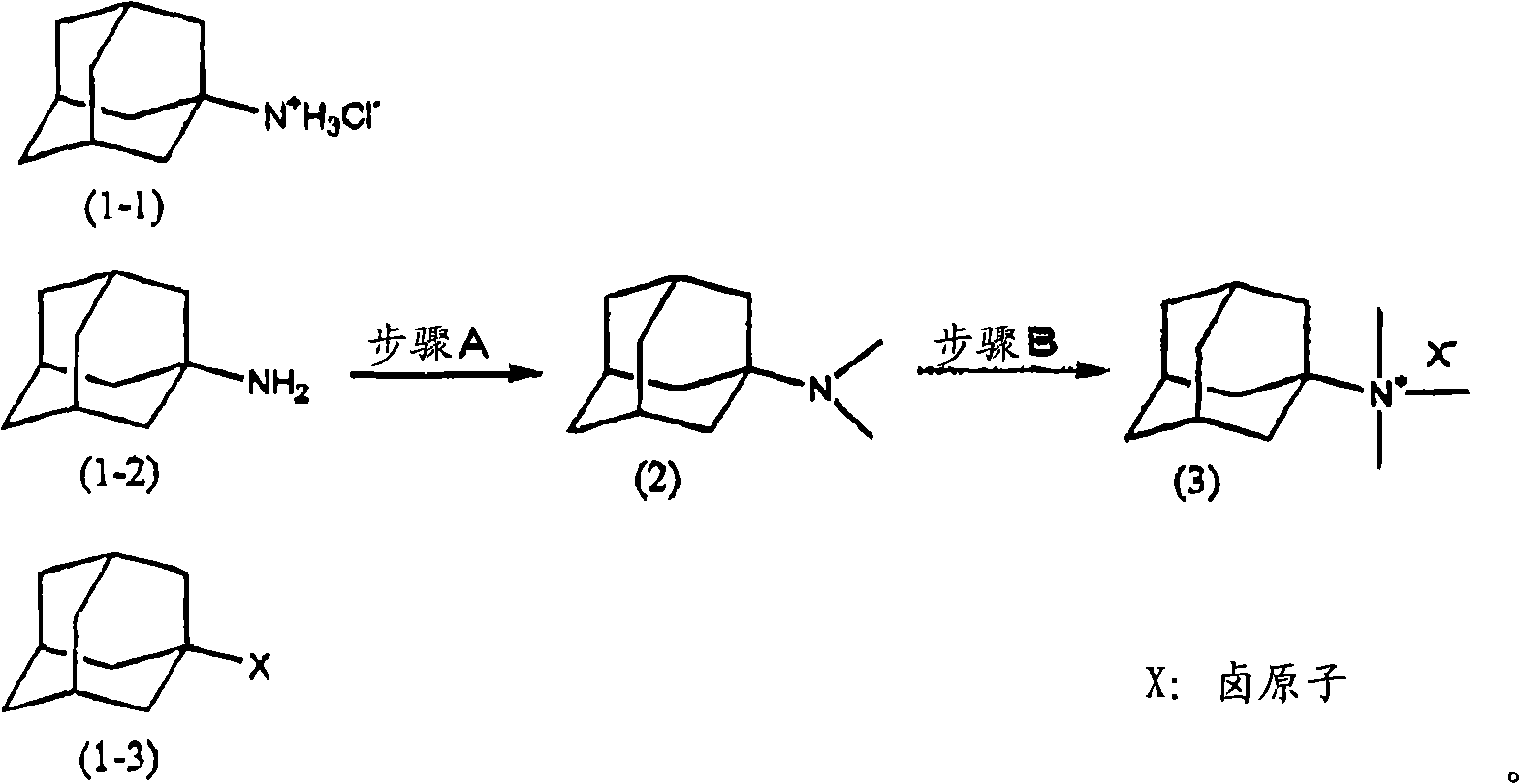
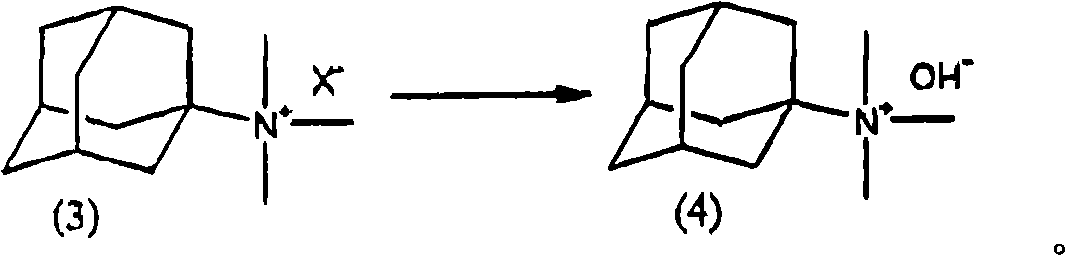
[0052] The preparation method of the quaternary ammonium salt having an adamantyl group of the present invention is characterized in that step A and step B are included in sequence.
[0053] [Step A]
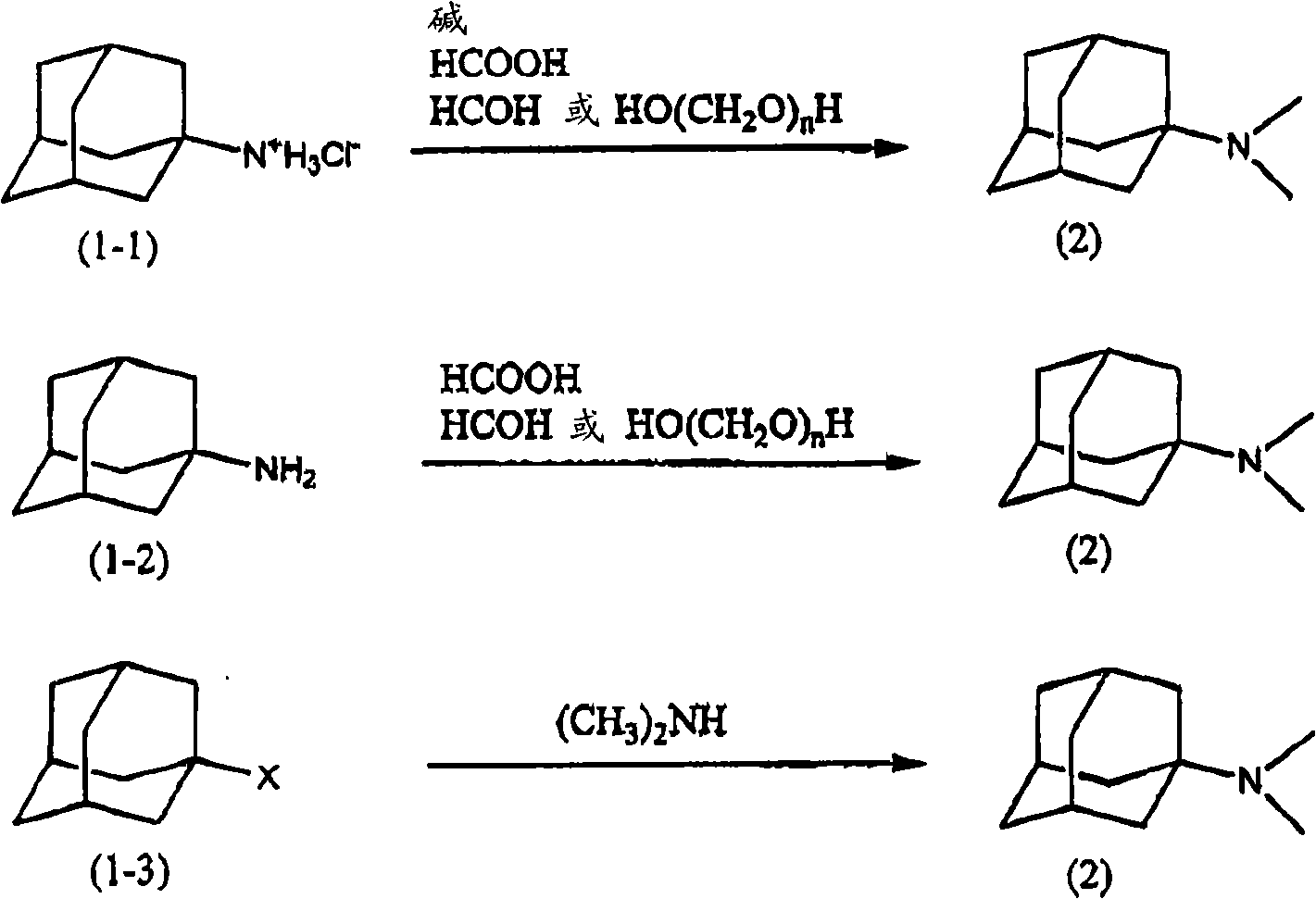
[0054] Step A is a step selected from the steps shown in the following reaction formula, including a manufacturing step selected from the following steps: when using amantadine hydrochloride [following formula (1-1)] as the starting material, dissolving in Relative permittivity is the reactant of bases such as amantadine hydrochloride and alkali metal hydroxide in the solvent a of 10.0~20.0 reacts with formic acid and formaldehyde or paraformaldehyde, forms 1-adamantane dimethylamine [following formula (2 )], or when using 1-aminoadamantane [following formula (1-2)] as a starting material, the 1-aminoadamantane dissolved in solvent a reacts with formic acid and formaldehyde or paraformaldehyde to form The step of 1-adamantane dimethylamine [following formula (2)], and then when...
Embodiment 1
[0142] Add 39.4 g of amantadine hydrochloride [FW: 187.11, 0.21 mol] and 200 mL of 2-propanol (relative dielectric constant: 18) into a 500 ml three-necked flask equipped with a reflux condenser, a thermometer, and a stirrer to dissolve. After raising the temperature of the solution to 50°C, 17 g of a 50% by mass aqueous sodium hydroxide solution was slowly added dropwise, stirred for 1 hour, and then 30 ml of a 97% by mass formic acid aqueous solution [FW: 46.03, 0.77 mol] was slowly added dropwise. While stirring, 85 g [FW: 30.03, 1.05 mol] of 37 mass % formaldehyde aqueous solution was dripped over 30 minutes. After completion of the dropwise addition, the temperature was raised to 80° C., and the reaction was performed for 3 hours. The reaction liquid was cooled, and it adjusted to pH 10 using 25 mass % sodium hydroxide aqueous solution. Add 250 ml of ethyl acetate, and separate the organic phase.
[0143] Add the fractionated crude 1-adamantyldimethylamine solution into...
Embodiment 2
[0145] 39.4 g of amantadine hydrochloride [FW: 187.11, 0.21 mol] and 200 mL of 2-propanol were added to a 500 ml three-necked flask equipped with a reflux condenser, a thermometer, and a stirrer, and dissolved. After raising the temperature of the solution to 50°C, 17 g of a 50% by mass aqueous sodium hydroxide solution was slowly added dropwise, stirred for 1 hour, and then 30 ml of a 97% by mass formic acid aqueous solution [FW: 46.03, 0.77 mol] was slowly added dropwise. While stirring, 85 g [FW: 30.03, 1.05 mol] of 37 mass % formaldehyde aqueous solution was dripped over 30 minutes. After completion of the dropwise addition, the temperature was raised to 80° C., and the reaction was performed for 3 hours. The reaction liquid was cooled, and it adjusted to pH 10 using 25 mass % sodium hydroxide aqueous solution. Add 250 ml of ethyl acetate, and separate the organic phase.
[0146] Add the fractionated crude 1-adamantyldimethylamine solution into a 500ml three-necked flask...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com