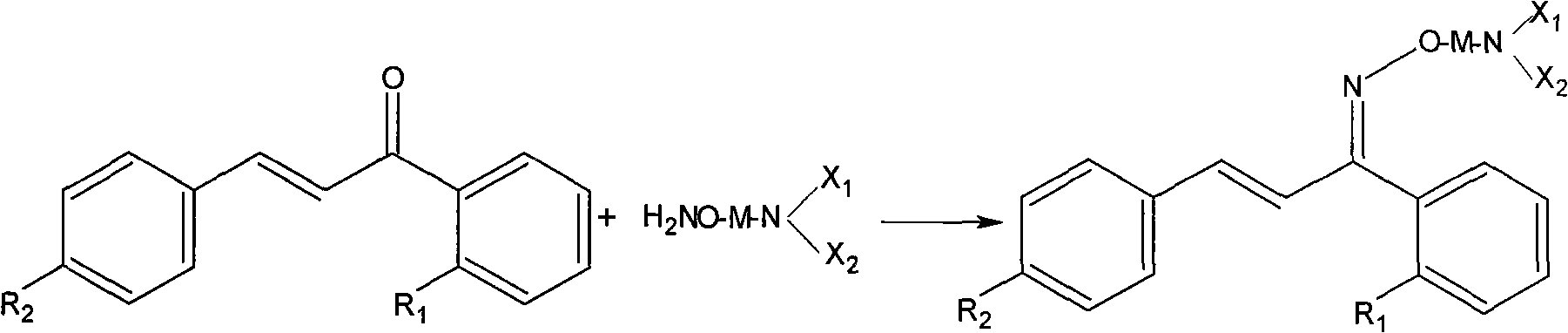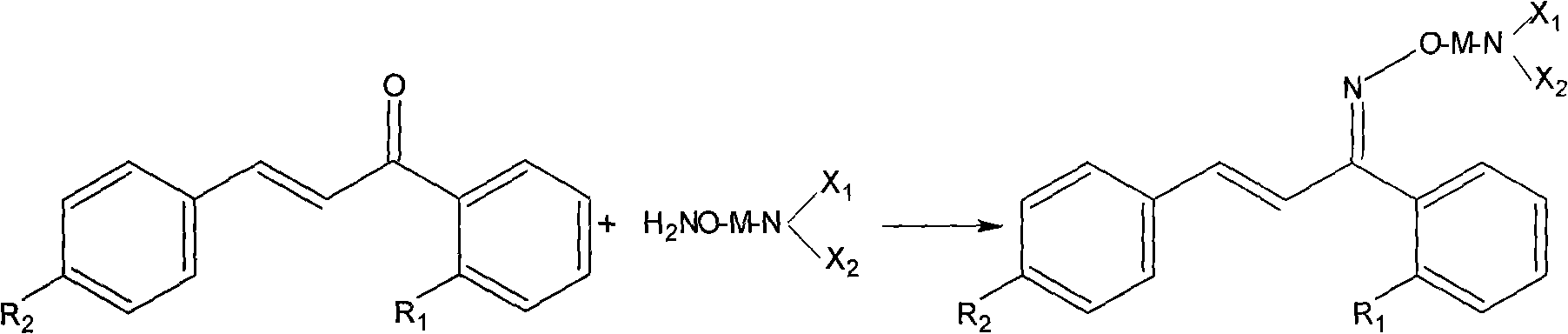Method for preparing derivatives of cis-oximes and oxime ethers
A technology of derivatives and oxime ethers, applied in the field of medicinal chemistry, can solve the problems of low yield, unfavorable industrial production, complicated operation, etc., and achieve the effect of good product quality, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 2'-Fluoro-4-hydroxychalcone-Z-oxime
[0025] In a 1L flask equipped with stirring, thermometer, and reflux condenser, add 2,-fluoro-4-hydroxychalcone (50.0g, 0.2mol), hydroxylamine hydrochloride (69.5g, 1.0mol), sodium acetate (65.6g , 0.8mol), 600mL absolute ethanol, pH4~6, react at 40℃ for 6h. The solvent was distilled off under reduced pressure to obtain a yellow-brown viscous substance, which was added with 1 L of water and 1 L of dichloromethane, separated into layers, and the organic layer was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain a pale yellow solid. 250ml of diethyl ether was crystallized to obtain 40.5g of white crystals with a melting point of 196-200°C and a yield of 75%. HPLC determination (0.02mol·L -1 Dipotassium hydrogen phosphate: acetonitrile: methanol: = 2:1:1, Z-type content > 85%)
[0026] 1 HNMR (400MHz, DMS O), δ (ppm) 6.30 (1H, d, H-C=) 6.70 (2H, d, H 3,5 )6.95(1H, d, H...
Embodiment 2
[0027] Example 2 2'-Fluoro-4-hydroxychalcone-Z-oxime
[0028] In a 1L flask equipped with stirring, thermometer and reflux condenser, add 2'-fluoro-4-hydroxychalcone (50.0g, 0.2mol), hydroxylamine hydrochloride (69.5g, 1.0mol), anhydrous potassium carbonate ( 110.6g, 0.8mol), 600mL absolute ethanol, pH4~6, reflux for 4h. The insoluble matter was filtered off, and the solvent was distilled off under reduced pressure to obtain a yellow-brown viscous substance. Add 1L of water and 1L of dichloromethane, separate layers, and dry the organic layer over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain a pale yellow solid. 250ml of diethyl ether was crystallized to obtain 45.2g of white crystals with a melting point of 196-200°C and a yield of 83.7%. HPLC determination (0.02mol L-1 dipotassium hydrogen phosphate: acetonitrile: methanol: = 2: 1: 1, Z-type content > 90%)
[0029] 1 HNMR (400MHz, DMSO), δ (ppm) 6.30 (1H, d, H-C=) 6.80 (2H, ...
Embodiment 3
[0030] Example 3 2'-Fluoro-4-hydroxychalcone-Z-oxime
[0031]In a 1L flask equipped with stirring, a thermometer, and a reflux condenser, add 2'-fluoro-4-hydroxychalcone (50.0g, 0.2mol), hydroxylamine hydrochloride (69.5g, 1.0mol), 600mL absolute ethanol, 1% sodium hydroxide ethanol solution to adjust the pH to 4-6, magnesium oxide 5g, reflux reaction for 8h. The solvent was distilled off under reduced pressure to obtain a yellow-brown viscous substance, which was added with 1 L of water and 1 L of dichloromethane, separated into layers, and the organic layer was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain a pale yellow solid. 250ml of diethyl ether was crystallized to obtain 38.9g of white crystals with a melting point of 196-200°C and a yield of 72%. HPLC determination (0.02mol·L -1 Dipotassium hydrogen phosphate: acetonitrile: methanol: = 2:1:1, Z-type content > 80%)
[0032] 1 HNMR (400MHz, DMSO), δ (ppm) 6.30 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com