Method for preparing aliphatic polycarbonates by catalyzing by metal cyanide coordination catalyst
A technology of coordination catalyst and polycarbonate, which is applied in the field of aliphatic polycarbonate catalyzed by metal cyanide coordination catalyst, which can solve the problem of high content of cyclic products, low molecular weight polycarbonates and low carbonate chain link content And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Preparation of metal cyanide coordination catalyst
[0072] Step 1: Add 0.80g K 3 Co(CN) 6 (0.0024mol) was dissolved in 50mL deionized water I, and 2mL (1.57g) of tert-butanol was added to obtain a mixed solution I'. The mixed solution I'was adjusted to pH<7 by adding aqueous hydrochloric acid solution, and the solution was uniform and transparent. Add to the zinc chloride aqueous solution (mixed solution II') formed by dissolving 4.0g (0.029mol) zinc chloride in 20mL deionized water II, stirring at 40°C for 24 hours, and suction filtration to obtain a semi-dry solid Filter cake
[0073] Step 2: Disperse the mixture of the filter cake obtained in the previous step and 0.5g zinc chloride (0.0037mol) in anhydrous tert-butanol with 2.0g 1-phenylimidazole (ie N-phenylimidazole, 0.0139mol) dissolved (20 mL), stirred at 60°C for 10 hours, and filtered with suction to obtain a white solid. The obtained white solid was re-dispersed in 40 mL of anhydrous tert-butanol, sti...
Embodiment 2
[0078] Example 2 Preparation of metal cyanide coordination catalyst
[0079] Same as Example 1, except that 2.1g of EO is added to the mixed solution I'of step 1. 20 PO 70 EO 20 (Pluronic P123, Aldrich), 4.2wt% of the weight (50g) of deionized water I. Finally, 1.5 g of a solid metal cyanide coordination catalyst was obtained.
[0080] Elemental analysis results: Zn: 19.4wt%; Co: 9.6wt%; Cl: 6.3wt%; C: 28.84wt%; H: 3.27wt%; N: 16.35wt%
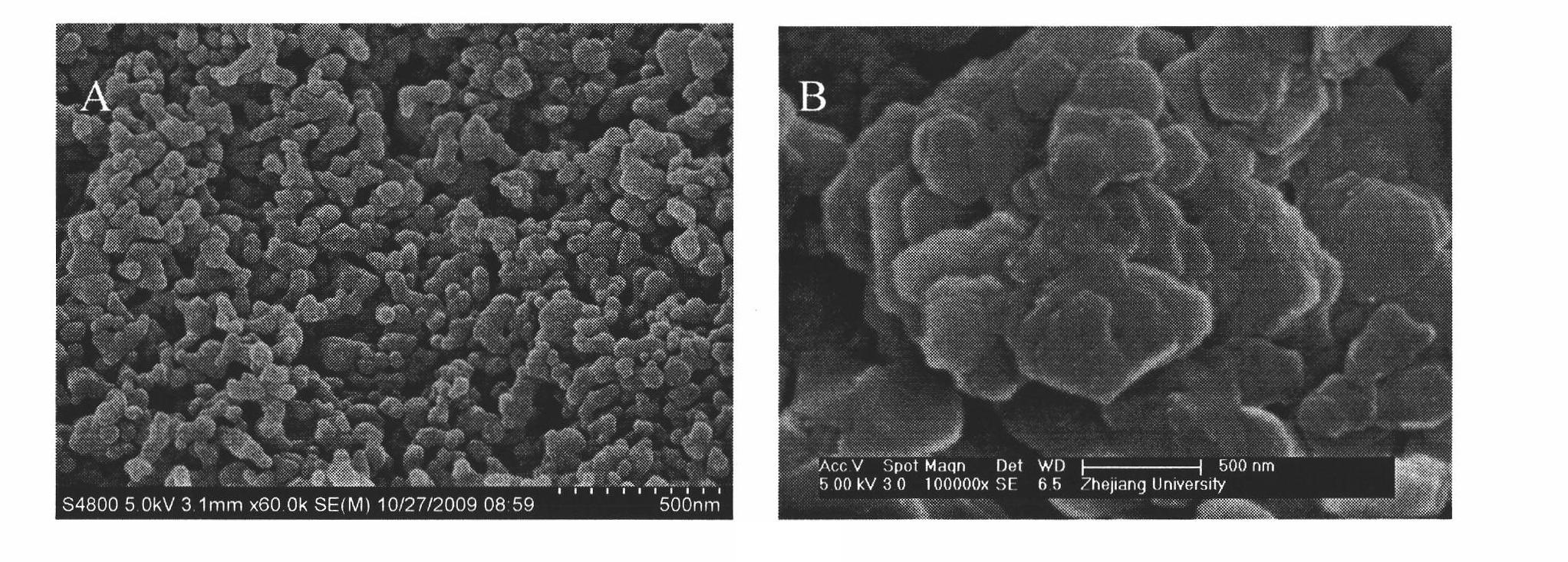
[0081] SEM observation (see figure 1 A): Spherical, with an average particle size less than 100nm;
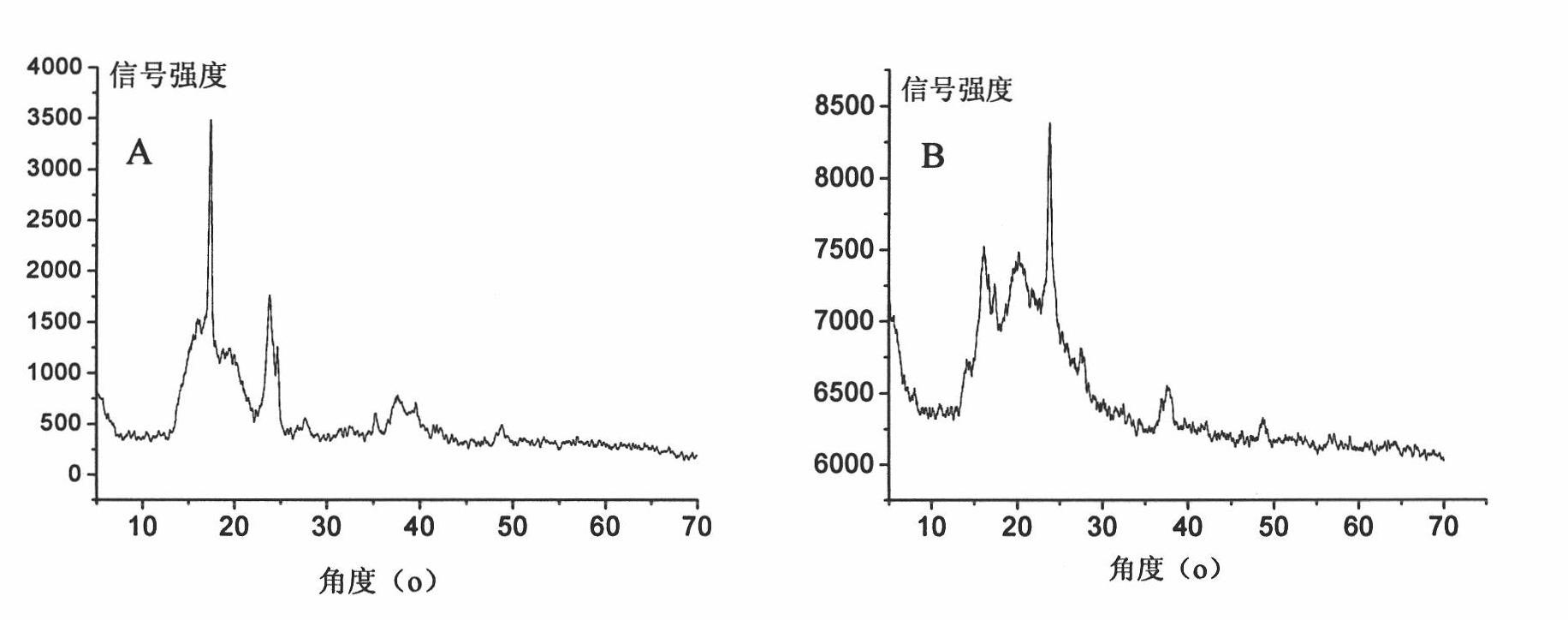
[0082] XRD results (see figure 2 A) Broad peaks are displayed in the range of 2θ=13~25°;
[0083] The average pore size measured by nitrogen adsorption method is 8nm.
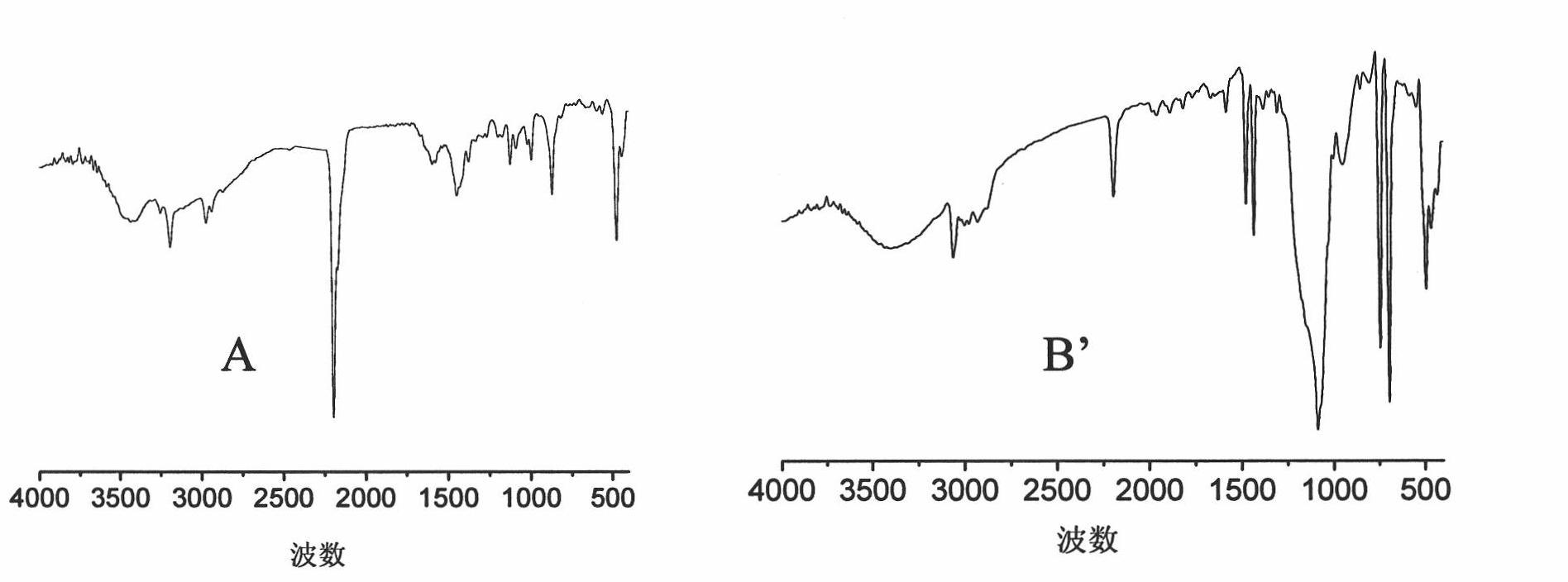
[0084] Infrared spectrum (see image 3 A) The 2294 and 472 wavenumber peaks are the characteristic infrared absorption peaks of the CN and Co-C bonds in the catalyst; the 1500 and 1200 wavenumber peaks indicate the presence of organic ligands in the catalyst.
Embodiment 3
[0085] Example 3 Preparation of metal cyanide coordination catalyst
[0086] Same as Example 1, except that in step 2, 1-phenylimidazole was replaced with equimolar diphenyl sulfoxide, anhydrous tert-butanol was replaced with an equal volume of anhydrous tetrahydrofuran, and the slurry was dispersed in anhydrous tetrahydrofuran. The slurry temperature is the reflux temperature of tetrahydrofuran. 1.8 g of a solid metal cyanide coordination catalyst was obtained.
[0087] Elemental analysis results: Zn: 19.2wt%; Co: 9.2wt%; Cl: 2.8wt%; C: 26.04wt%; H: 1.03wt%; N: 15.78wt%.
[0088] SEM observation (see figure 1 B) It is flake-shaped, and the thickness of the flake: 20-40nm;
[0089] XRD results show broad peaks in the range of 2θ=13~25°;
[0090] The average pore size measured by nitrogen adsorption method is 45nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average pore size | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com