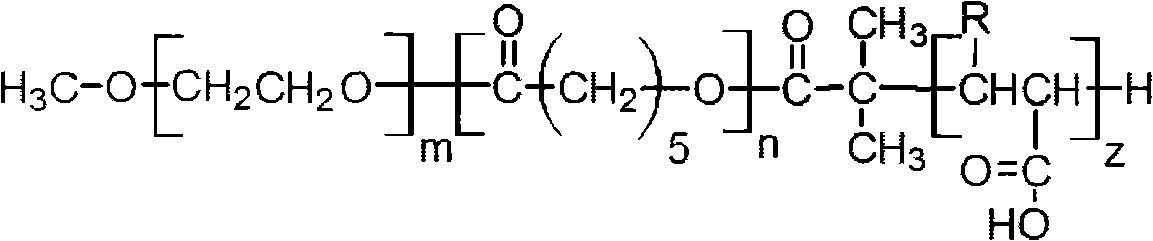Polyethylene glycol momomethyl ether-polycaprolactone-polyacrylic acid derivatives, and preparation and application thereof
A technology of monomethyl ether and polyethylene glycol, applied in the field of polyethylene glycol monomethyl ether-polycaprolactone-polyacrylic acid derivatives and their preparation and application, to improve solubility and oral bioavailability , to avoid the effect of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
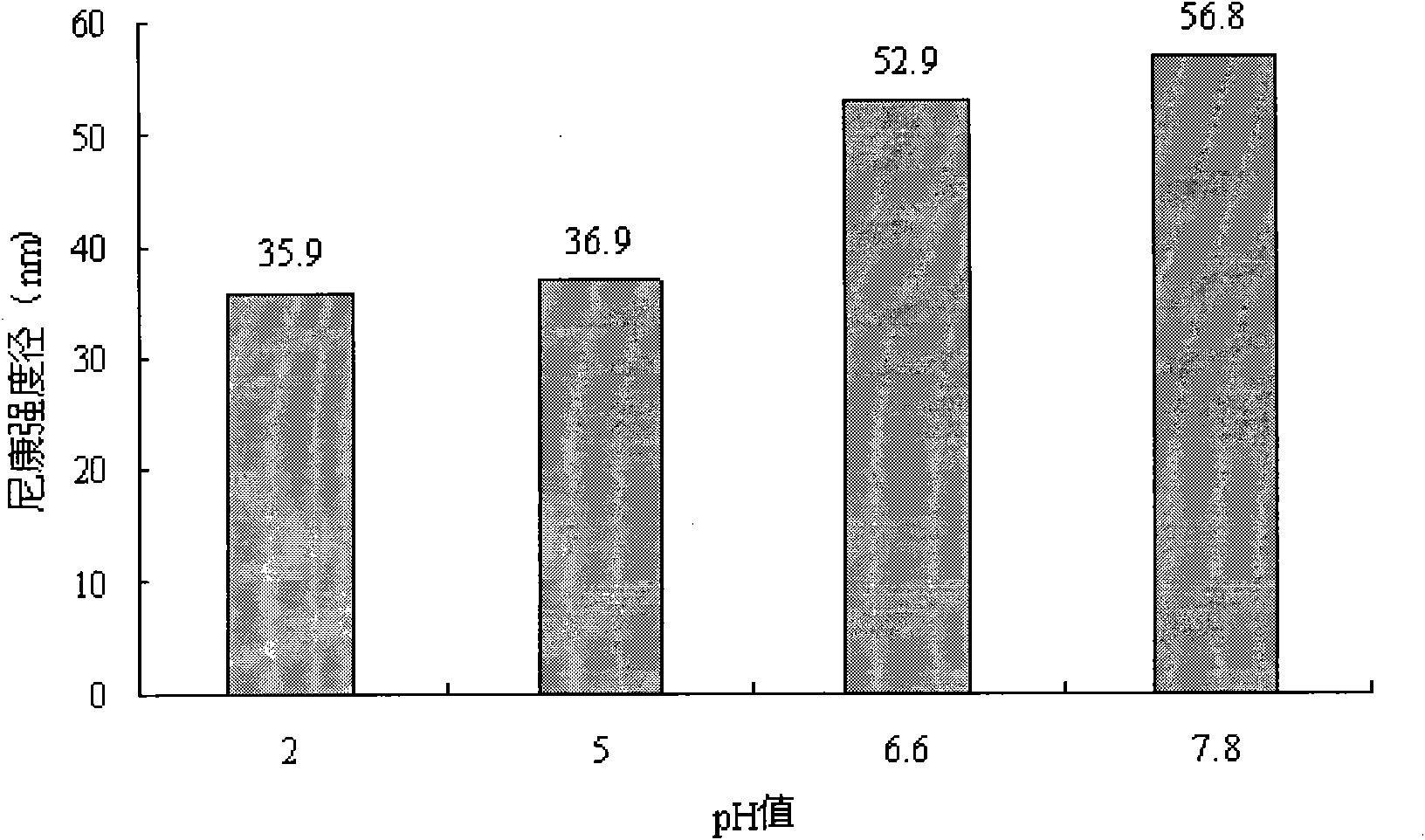
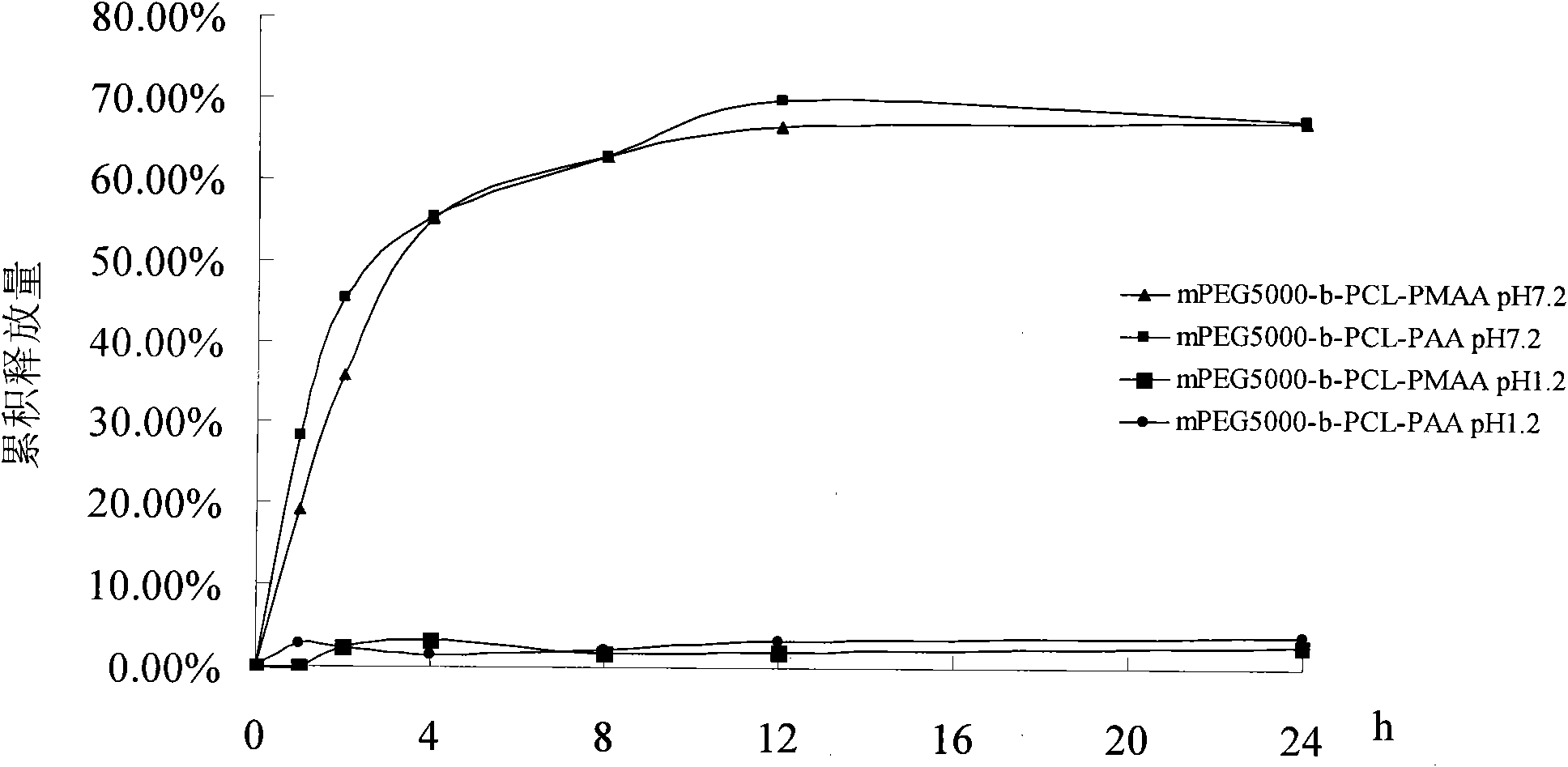
Image
Examples
Embodiment 1
[0059] Example 1: mPEG 5000 -Preparation of b-PCL-b-PAA block copolymer
[0060] Materials and Methods
[0061] ε-caprolactone (ε-CL): purchased from Sigma-Aldrich Shanghai Trading Co., Ltd., currently used in CaH 2 The reaction was carried out after drying in medium for 24 hours and distillation. Monomethyl ether of polyethylene glycol (mPEG): purchased from Sigma-Aldrich Shanghai Trading Co., Ltd., with a molecular weight of 5000 Da. Currently, the reaction is carried out after redistillation in freshly steamed toluene. Stannous octoate was purchased from Sigma-Aldrich Shanghai Trading Co., Ltd.
[0062] (1) Synthesis of mPEG-b-PCL block copolymer:
[0063] Using the ring-opening polymerization method, prepare mPEG with a mass ratio of 5:3, 5:5, 5:7 or 5:9, respectively 5000 -b-PCL. A) Take 5g of mPEG (5000Da) and 3g of ε-CL to prepare 5:3 mPEG 5000 -b-PCL; B) Take 5g of mPEG (5000Da) and 5g of ε-CL to prepare 5:5 mPEG 5000 -b-PCL; C) Take 5g of mPEG (5000Da) and 7g...
Embodiment 2
[0075] Example 2: mPEG 2000 -Preparation of b-PCL-b-PMAA block copolymer
[0076] Materials and Methods
[0077] ε-caprolactone (ε-CL): purchased from Sigma-Aldrich Shanghai Trading Co., Ltd., currently used in CaH 2 The reaction was carried out after drying in medium for 24 hours and distillation. Monomethyl ether of polyethylene glycol (mPEG): purchased from Sigma-Aldrich Shanghai Trading Co., Ltd., with a molecular weight of 5000 Da. Currently, the reaction is carried out after redistillation in freshly steamed toluene. Stannous octoate was purchased from Sigma-Aldrich Shanghai Trading Co., Ltd.
[0078] (1) Synthesis of mPEG-b-PCL block copolymer:
[0079] Using the ring-opening polymerization method, prepare mPEG with a mass ratio of 5:3, 5:5, 5:7 or 5:9, respectively 2000 -b-PCL. A) Take 5g of mPEG (2000Da) and 3g of ε-CL to prepare 5:3 mPEG 2000 -b-PCL; B) Take 5g of mPEG (2000Da) and 5g of ε-CL to prepare 5:5 mPEG 2000 -b-PCL; C) Take 5g of mPEG (2000Da) and 7...
Embodiment 3
[0088] Embodiment 3: Preparation of docetaxel polymer micelles by dialysis
[0089] Accurately weigh 50 mg successively to get the mPEG obtained in Example 1 5000 -b-PCL-b-PAA, each dissolved in 2ml tetrahydrofuran; at the same time, tetrahydrofuran was used as a solvent to configure a 1mg / mL docetaxel solution, and 2mL docetaxel solution was added to the configured polymer solution to obtain Drug carrier solution with a drug loading ratio of 4%. After 4 hours of magnetic stirring at room temperature, under strong stirring, 6 mL of redistilled water was added to the system at a rate of one drop per 10 seconds for hydration. After the addition was complete, the stirring was continued for 4 hours. In a dialysis bag, dialyze in double distilled water for 24 hours, and filter with a 0.45 μm microporous membrane after dialysis.
[0090] Table 3 for four different mPEG 5000 -Drug loading of b-PCL-b-PAA using dialysis method:
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com