Method for extracting lithium and other alkali metal elements from lepidolite mineral
A technology of metal elements and lepidolite, applied in the direction of improving process efficiency, can solve problems such as equipment corrosion, low production efficiency, environmental pollution, etc., and achieve the effect of eliminating environmental impact, reducing energy consumption and cost, and reducing processing procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
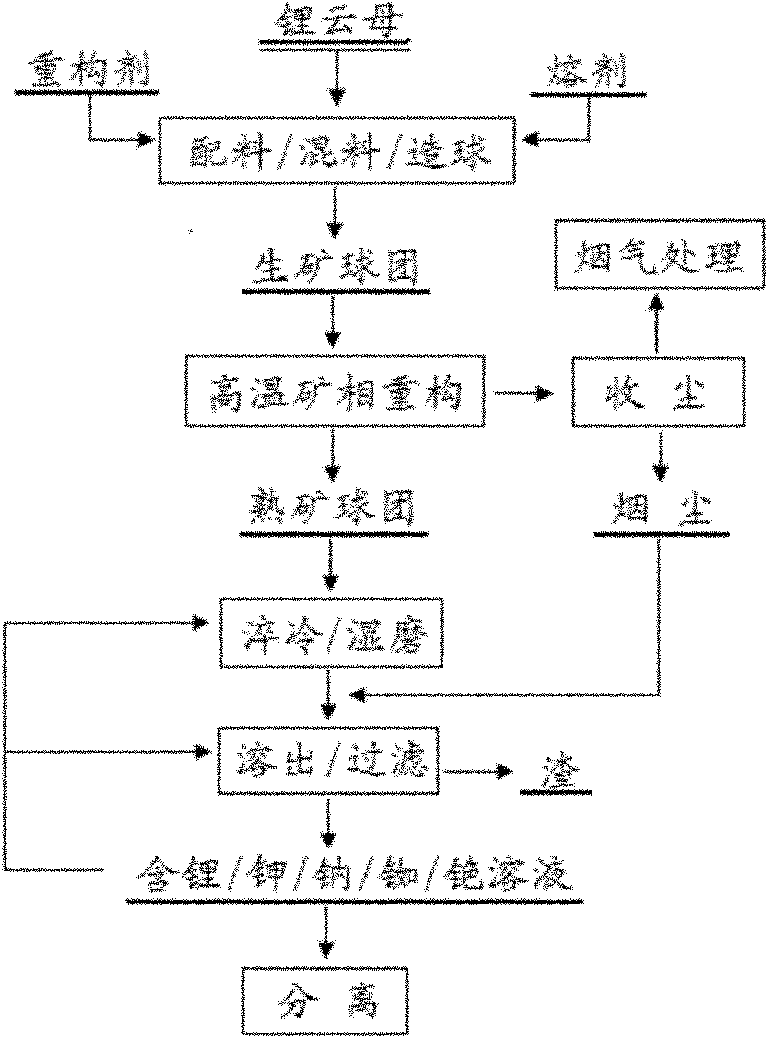
[0049] The main components and content (%) of lepidolite ore are: Li 2 O 3.6; (K, Na) 2 O 8.5; (Rb, Cs) 2 O 1.4; Al 2 o 3 22.5; SiO 2 58.0; F 4.5.
[0050] Mix lepidolite, calcium chloride, and sodium chloride at a mass ratio of 1.00:0.28:0.42 to make ~25mm raw ore pellets; roast the above-mentioned raw ore pellets at 850°C for 50 minutes, and cool the furnace gas. Collect smoke and dust and purify; after roasting, the cooked ore pellets are quenched with an aqueous solution, and then wet ball milled, together with the smoke and dust, use an aqueous solution to dissolve lithium, potassium, rubidium and cesium at room temperature, and obtain lithium, potassium, sodium, rubidium and cesium mixed chloride solution in water. Silicon and aluminum in the slag are mainly composed of (Ca, Na)O·(Al, Si) 2 o 3 2SiO2 2 and CaO·SiO 2 exists, fluorine is fixed as CaF 2 .
[0051] The dissolution yields (%) of each main component are: Li 91.2; K 93.3; Rb+Cs 87.1;
[0052] The f...
Embodiment 2
[0054] The main components and content (%) of lepidolite ore are: Li 2 O 4.5; (K, Na) 2 O 9.2; (Rb, Cs) 2 O 1.6; Al 2 o 3 28.2; SiO 2 47.0; F 5.2
[0055] Mix lepidolite, calcium sulfate, and sodium sulfate at a mass ratio of 1.00:0.72:0.48 to make ~25mm raw ore pellets; roast the above raw ore pellets at 950°C for 30 minutes, cool the furnace gas, and collect smoke and dust and purification treatment; after roasting, the cooked ore pellets are quenched with an aqueous solution, and then wet ball milled, together with the dust, an aqueous solution is used to dissolve lithium, potassium, rubidium and cesium at 70°C to obtain lithium, potassium, sodium, rubidium and cesium. Mix the sulfate solution. Silicon and aluminum in the slag are mainly composed of (Ca, Na)O·(Al, Si) 2 o 3 2SiO2 2 Existence, fluorine is fixed as CaF2.
[0056] The dissolution yield (%) of each main component is respectively: Li 93.1; K 94.4; Rb+Cs 88.7;
[0057] The fixation rate of fluorine in ...
Embodiment 3
[0059] The main components and content (%) of lepidolite ore are: Li 2 O 5.4; (K, Na) 2 O 8.1; (Rb, Cs) 2 O 1.7; Al 2 o 3 24.6; SiO 2 50.2; F 6.4
[0060] Mix lepidolite, calcium carbonate, and sodium hydroxide at a mass ratio of 1.00:0.55:0.45 to make ~25mm raw ore pellets; roast the above-mentioned raw ore pellets at 900°C for 20 minutes, cool the furnace gas, and collect Soot and purification treatment: After roasting, the cooked ore pellets are quenched with aqueous solution, and then wet ball milled, together with the soot, they are dissolved with an aqueous solution at 50°C to dissolve lithium, potassium, rubidium and cesium, etc., to obtain lithium, potassium, sodium, rubidium and cesium mixed hydroxide solution. The silicon and aluminum in the molten slag are mainly CaO·Al 2 o 3 2SiO2 2 , (Ca, Na)O · (Al, Si) 2 o 3 2SiO2 2 and CaO·SiO 2 exists, fluorine is fixed as CaF 2 .
[0061] The dissolution yield (%) of each main component is respectively: Li 92.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com