Method for synthesizing 3-methylpyridine
A technology of picoline and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of 3-picoline by-products, which have not been fundamentally solved, and affect the yield, etc., to achieve lower temperature, high buffering effect, The effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
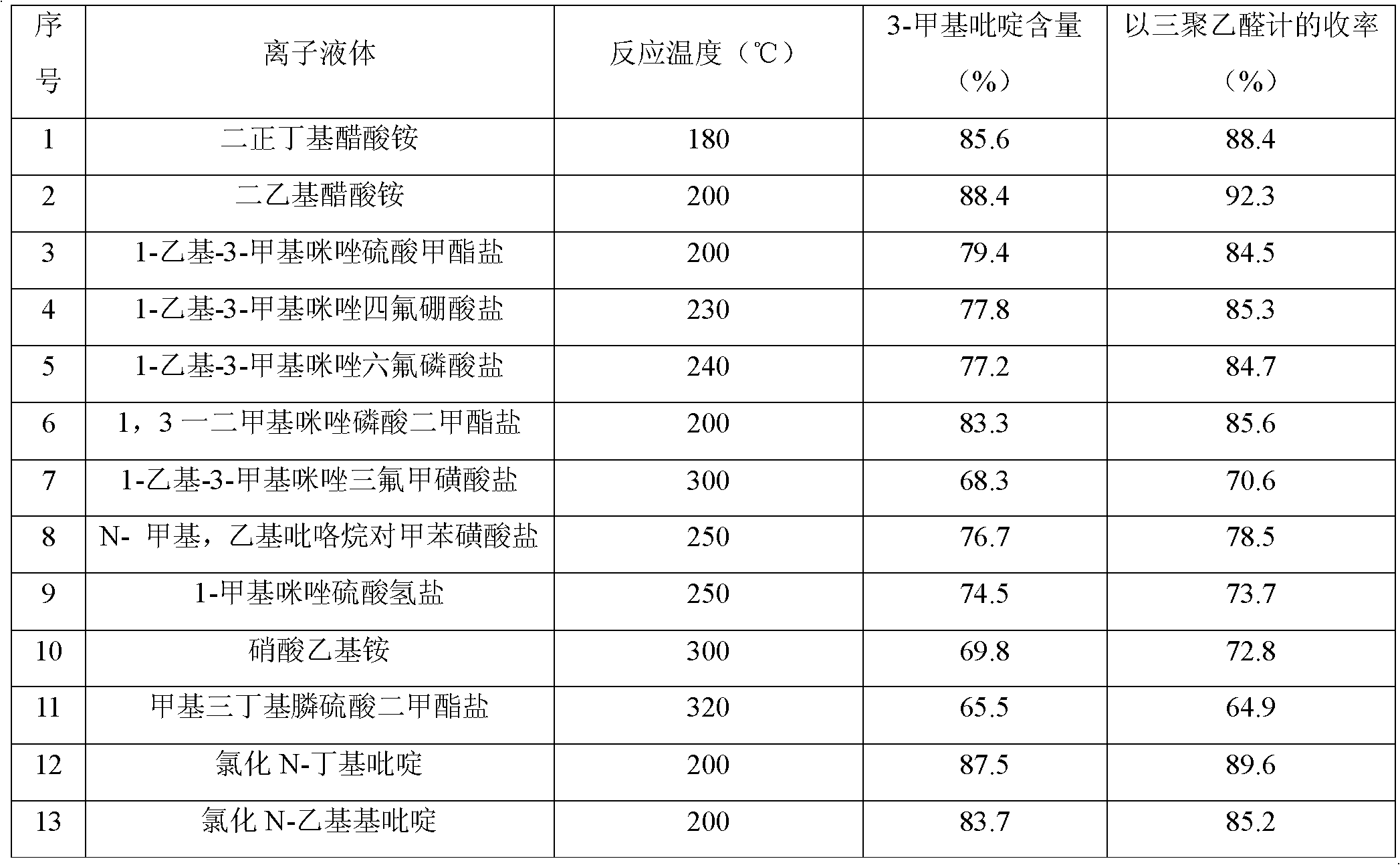
[0016] Weigh 250 g of di-n-butylammonium acetate ionic liquid and set aside.
[0017] Connect the emptying port of the autoclave to the vacuum pump with a rubber tube, insert the feed tube of the autoclave into the weighed ionic liquid, open the needle valve at the feed port, and vacuum the weighed ionic liquid Put it into the autoclave (if the ionic liquid solidifies, it can be heated slightly). After suction is complete, close the autoclave emptying needle valve and close the feeding needle valve.
[0018] Turn on the autoclave to stir, set the stirring speed to 400r / min, set the heating temperature to 200°C, set the heating voltage to 200V, and start heating.
[0019] Weigh 122.5g of paraldehyde, 65g of urotropine, 130g of distilled water, and 150g of ethanol, mix well and set aside. When the temperature inside the autoclave rises to 200°C. Turn on the liquid phase pump, adjust the feed flow rate to 7.8g / min, and control the feeding of about 460g into the autoclave for 2...
Embodiment 2
[0023] Weigh 250 g of diethylammonium acetate ionic liquid and set aside.
[0024] Connect the emptying port of the autoclave to the vacuum pump with a rubber tube, insert the feed tube of the autoclave into the weighed ionic liquid, open the needle valve at the feed port, and vacuum the weighed ionic liquid Put it into the autoclave (if the ionic liquid solidifies, it can be heated slightly). After suction is complete, close the autoclave emptying needle valve and close the feeding needle valve.
[0025] Turn on the autoclave to stir, set the stirring speed to 400r / min, set the heating temperature to 200°C, set the heating voltage to 200V, and start heating.
[0026] Weigh 122.5g of paraldehyde, 65g of urotropine, 130g of distilled water, and 150g of ethanol, mix well and set aside. When the temperature inside the autoclave rises to 200°C. Turn on the liquid phase pump, adjust the feed flow rate to 7.8g / min, and control the feeding of about 467.5g into the autoclave for 2 ...
Embodiment 3
[0030] Weigh 2000 g of diethylammonium acetate ionic liquid and set aside.
[0031] Weigh 1225g of paraldehyde, 650g of urotropine, 1300g of distilled water, and 1500g of ethanol, mix well and set aside.
[0032] Open pipe reactor heat transfer oil heating system, set the heating temperature to 250°C. When the temperature of the hot oil system reaches 250°C, turn on the ionic liquid high-pressure metering pump and adjust the feed flow rate to 4.8g / min. When the pressure in the reaction tube reaches 3.0MPa, open the outlet valve of the pipeline reactor to control the reaction pressure to about 3.0MPa.
[0033] Turn on the high-pressure metering pump for the reaction solution, and adjust the feed flow rate to 10 g / min. Adjust the opening of the outlet valve of the pipeline reactor to control the reaction pressure at about 3.0MPa.
[0034] The length of the pipe reactor is 25m, the inner diameter of the pipe is 4mm, and the residence time is about 20min.
[0035] The feed liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com