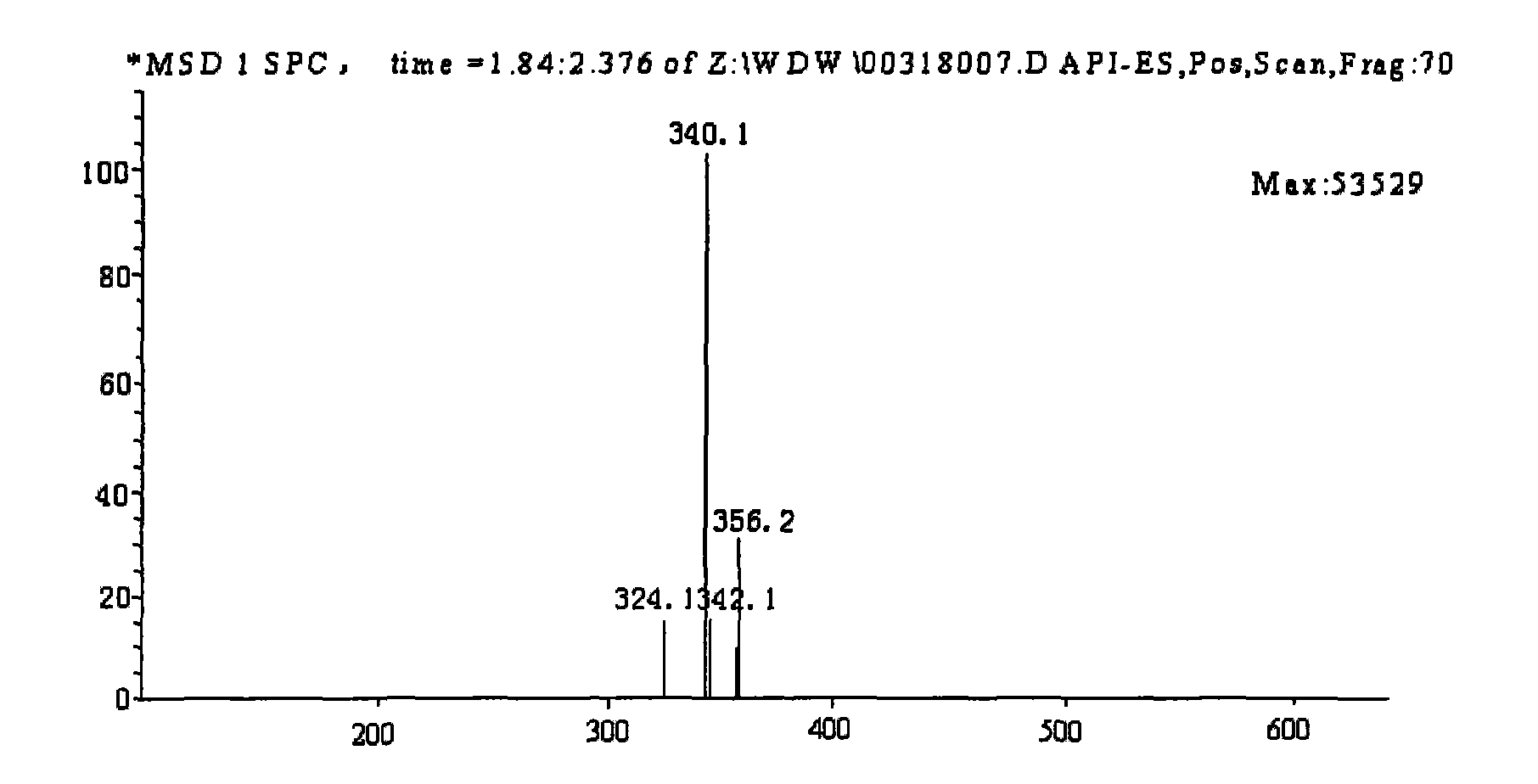
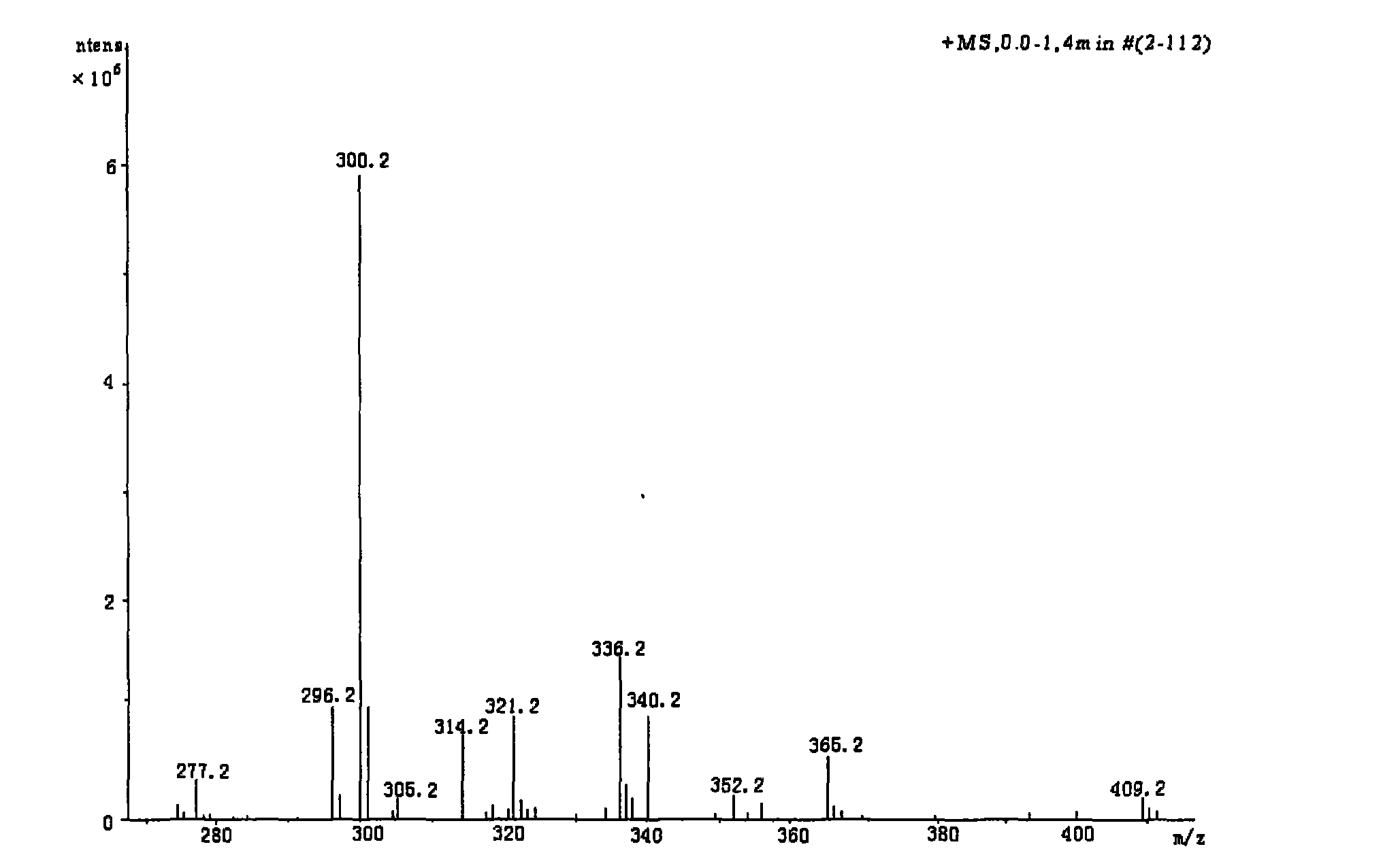
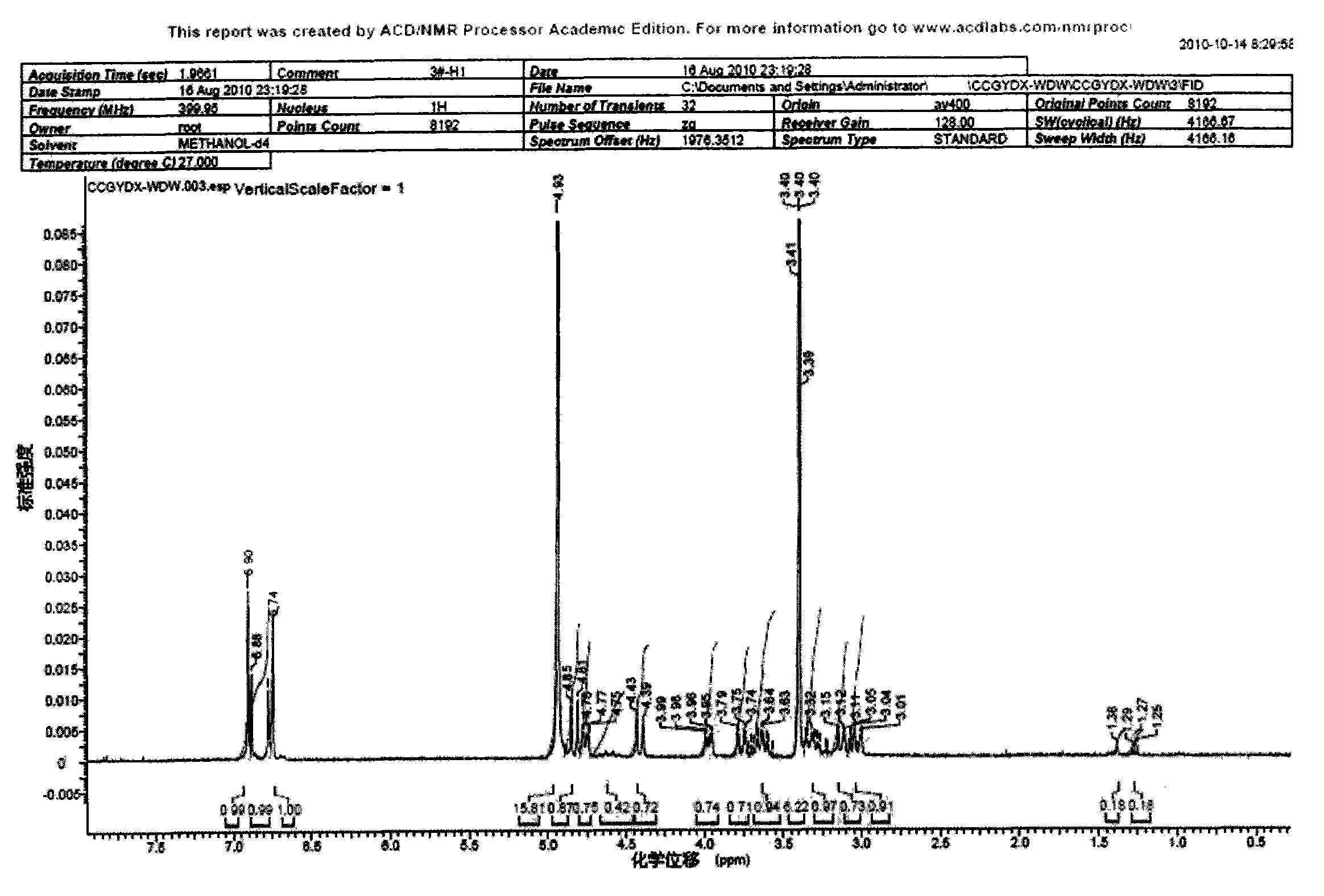
Method for preparing coptisine from coptis mixed alkaloid based on common basic structural characteristic
A basic structure and alkaloid technology, applied in the direction of organic chemistry, can solve the problems of easily forming only one ring-closing product, removing one aldehyde group by-product, and many steps in the total synthesis of coptisine, so as to save purification The effect of steps, strong activity, and simple and easy-to-operate preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of mixed alkaloids from Tetrahydrocoptis Rhizoma
[0048] Weigh 1 g of Coptis rhizome mixed alkaloids into a 100ml three-necked bottle, add 25ml of ethanol aqueous solution with a mass fraction of 70% ethanol, heat and dissolve to reflux at 80°C, the concentration of Coptis rhizome mixed alkaloids in ethanol aqueous solution is 0.04g / mL, add no Potassium carbonate water 1.6g, the mass ratio of anhydrous potassium carbonate and Coptidis rhizome mixed alkaloid is 1.6: 1, then every 5min is divided into three equal amounts and adds the sodium borohydride that total amount is 0.12g and reacts, and described sodium borohydride The mass ratio of mixed alkaloids with Coptidis rhizome was 0.12:1. After stirring and reacting at room temperature for 4 hours, suction filtration was performed to filter out the insoluble matter, which was recrystallized from benzene to obtain 0.83 g of mixed alkaloids of Coptidis rhizome with a yield of 84%.
Embodiment 2
[0049] Example 2: Preparation of mixed alkaloids of Tetrahydrocoptis Rhizoma
[0050] Weigh 1 g of the mixed alkaloids of Coptis chinensis into a 100 ml three-necked bottle, add 40 ml of ethanol aqueous solution with a mass fraction of 80% ethanol, heat and dissolve to reflux at 80°C, the concentration of the mixed alkaloids of Coptis chinensis in the aqueous ethanol solution is 0.025 g / mL, add Potassium carbonate water 3g, the mass ratio of anhydrous potassium carbonate and Coptidis rhizome mixed alkaloids is 3: 1, divides three times every interval 5min equal amount and adds the sodium borohydride that total amount is 0.3g to react, described sodium borohydride The mass ratio of mixed alkaloids with Coptidis rhizome was 0.3:1, stirred and reacted at room temperature for 6 hours, then suction filtered to filter out insoluble matter, which was recrystallized from benzene to obtain 0.88 g of mixed alkaloids of Coptidis rhizome with a yield of 87%.
Embodiment 3
[0051] Example 3: Preparation of mixed alkaloids from Tetrahydrocoptis Rhizoma
[0052] Weigh 1g of the mixed alkaloids of Coptidis rhizome in a 100ml three-neck bottle, add 50ml of ethanol aqueous solution with a mass fraction of 90% ethanol, heat and dissolve the mixed alkaloids of Coptidis rhizome with aqueous ethanol to reflux at 80°C, and the mixed alkaloids of Coptidis rhizome in the aqueous ethanol solution The concentration is 0.02g / mL, add 4g of anhydrous potassium carbonate, the mass ratio of anhydrous potassium carbonate and Coptidis rhizome mixed alkaloids is 4:1, then add a total of 0.6g of sodium borohydride in equal amounts three times at intervals of 5min Carry out the reaction, the mass ratio of sodium borohydride and Coptidis rhizome mixed alkaloids is 0.6:1, stir and react at room temperature for 8 hours, after the reaction is completed, filter with suction, filter out the insoluble matter, and recrystallize the insoluble matter through benzene to obtain Tetr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com