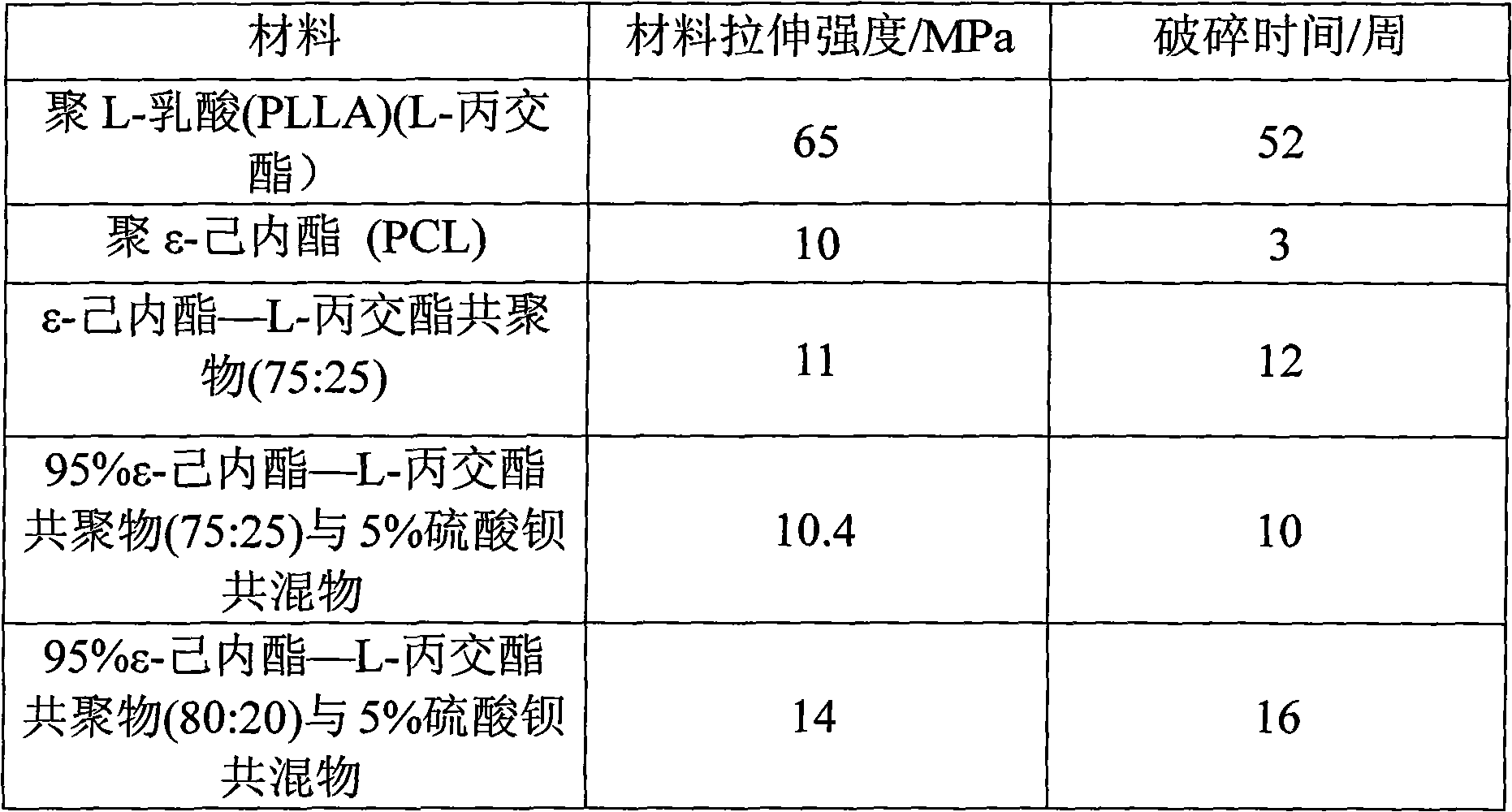
Degradable double-layer compound ureteral stent tube
A ureteral stent tube, double-layer composite technology, applied in stents, catheters, medical science and other directions, can solve the problems of unbearable pain, delayed extubation, low molecular weight, etc., to reduce pain and burden, prevent ureteral stenosis, biological phase Good capacitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Put 180 grams of ε-caprolactone monomer and 320 grams of L-lactide monomer after dehydration and purification into a 2500ml reaction flask, and then add the ε-caprolactone monomer and L-lactide monomer. A stannous octoate catalyst containing 0.01% of the total mass of the ester monomer was reacted at 150°C for 6 hours under vacuum conditions to obtain 500 g of elastic copolymer. The copolymer was dissolved in 2000 ml of acetone and precipitated with 4000 ml of ethanol. The material is dried in a vacuum dryer at 50°C for 48 hours to obtain an L-lactide / ε-caprolactone copolymer elastomer material. The molecular weight of the product was determined by gel permeation chromatography (GPC) and the weight average molecular weight was 230,000. 1 The H-spectrum measurement shows that the molar ratio of L-lactide to ε-caprolactone structural unit in the polymer is 75:25. The L-lactide / ε-caprolactone copolymer is determined by differential scanning calorimetry. Glass tran...
Embodiment 2
[0025] Example 2 Put 150 grams of ε-caprolactone monomer and 350 grams of L-lactide after dehydration and purification in a 2500 ml reaction flask, and then add the ε-caprolactone monomer and L-lactide monomer. The total mass of 0.01% stannous octoate catalyst was reacted at 150°C for 6 hours under vacuum to obtain 500 g of elastic copolymer. The copolymer was dissolved in 2000 ml of acetone and precipitated with 4000 ml of ethanol. The L-lactide / ε-caprolactone copolymer elastomer material is obtained by drying in a desiccator at 50°C for 48 hours. The molecular weight of the product is determined by Gel Permeation Chromatography (GPC). The weight average molecular weight is 455,000. 1 H-spectrum measurement shows that the molar ratio of L-lactide to ε-caprolactone structural unit in the polymer is 80:20. The L-lactide / ε-caprolactone copolymer is determined by differential scanning calorimetry. Glass transition temperature (T g ) Is 11°C. A solution blending method is used to ...
Embodiment 3
[0028] Example 3 Put 150 grams of ε-caprolactone monomer and 350 grams of L-lactide after dehydration and purification in a 2500 ml reaction flask, and then add the ε-caprolactone monomer and L-lactide monomer. The total mass of 0.01% stannous octoate catalyst was reacted at 150°C under vacuum for 5 hours to obtain 500 g of elastic copolymer. The copolymer was dissolved in 2000 ml of acetone and precipitated with 4000 ml of ethanol. The L-lactide / ε-caprolactone copolymer elastomer material is obtained by drying in a desiccator at 50°C for 48 hours. The molecular weight of the product is determined by Gel Permeation Chromatography (GPC). The weight average molecular weight is 100,000. 1 The H-spectrum measurement showed that the molar ratio of L-lactide to ε-caprolactone structural unit in the polymer was 77:23. The L-lactide / ε-caprolactone copolymer was determined by differential scanning calorimetry. Glass transition temperature (T g ) Is 7°C. A solution blending method is us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com