Method to synthesize quinuclidine hydrochloride
A technology of quinuclidine hydrochloride and synthesis method, which is applied in the synthesis of quinucline hydrochloride, in the field of quinuclidine hydrochloride, and can solve problems such as limiting the feasibility of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
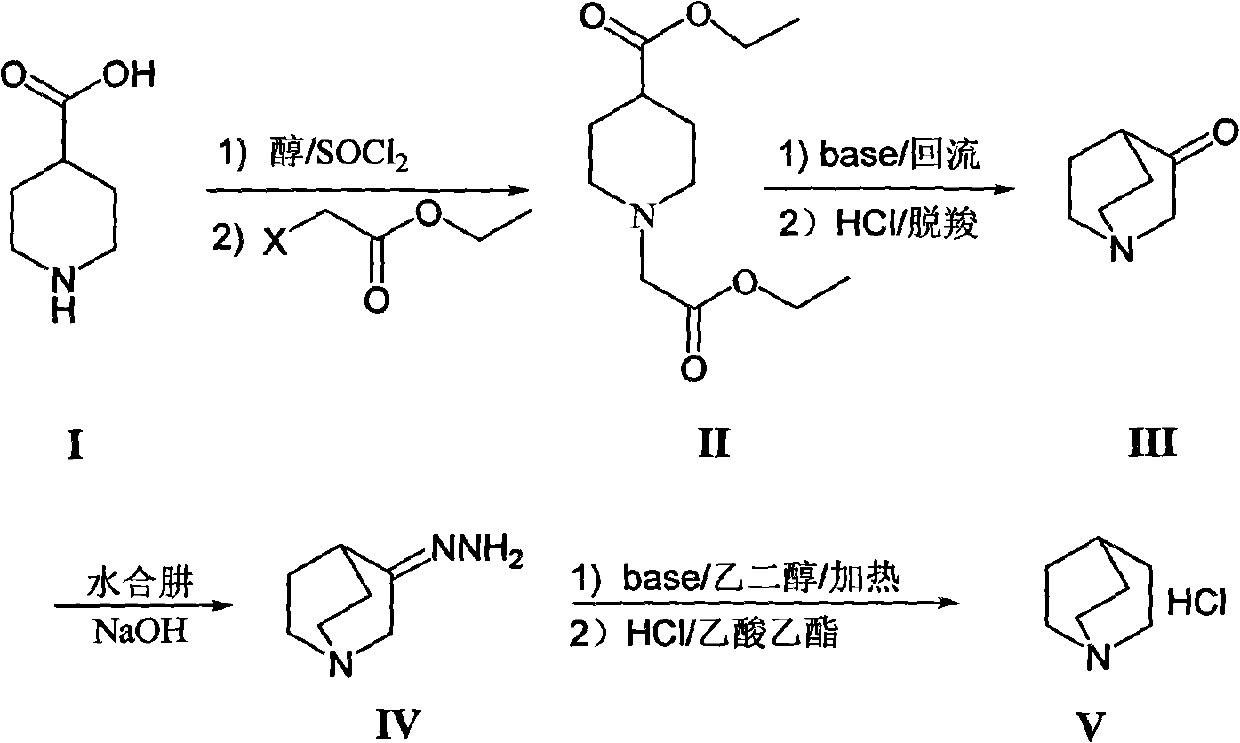
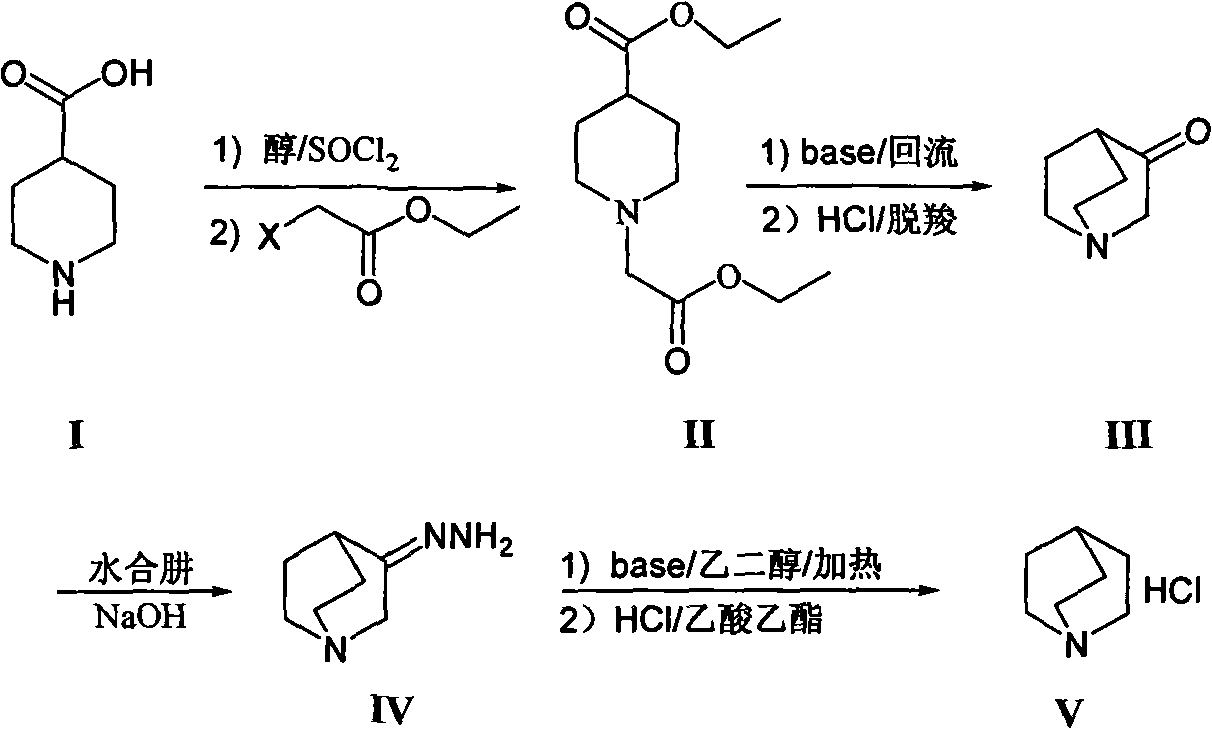
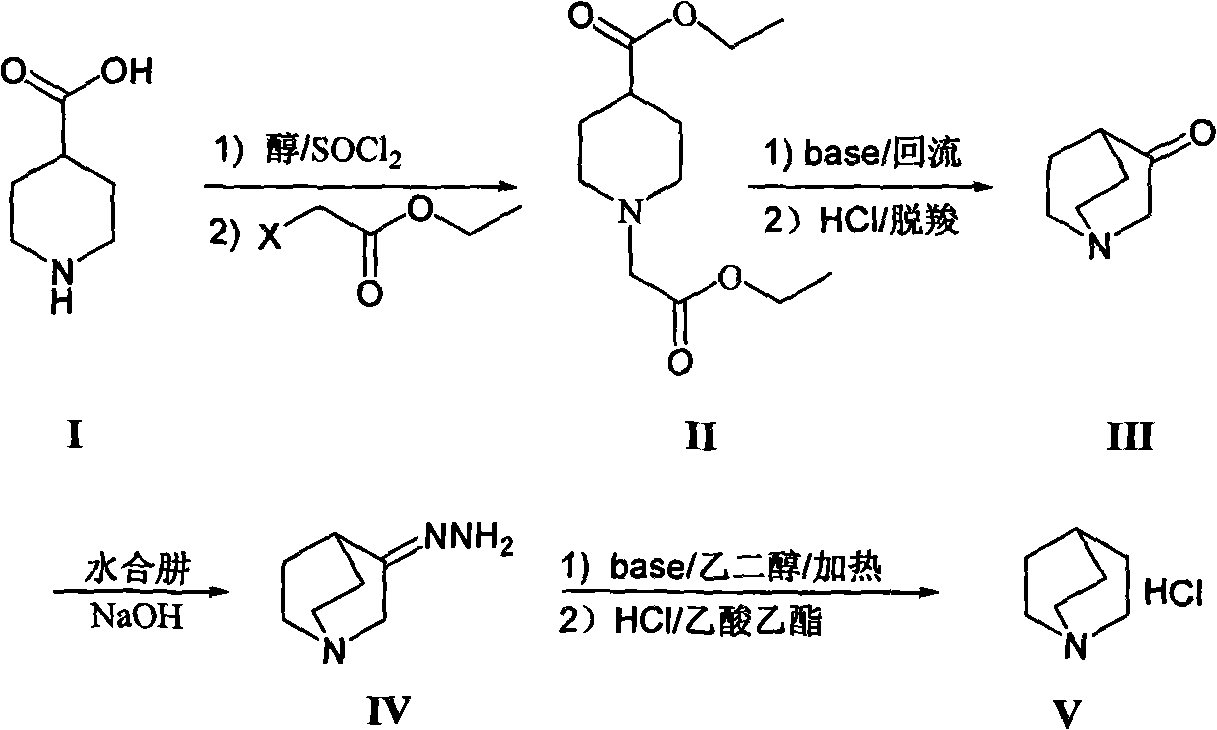
[0007] The synthesis of 1-ethoxycarbonyl ethyl-4-piperidinecarboxylic acid ethyl ester (II) is realized through the following steps:
[0008] 1) Method 1:
[0009] Throw 400g of 4-piperidinecarboxylic acid (I) into a 10L three-necked flask, and add an anhydrous alcohol solvent, which is 4L of anhydrous ethanol in this example. Another 2 mL of N,N-dimethylformamide was added, the temperature was controlled below 30°C, and 46.1 g of thionyl chloride was added dropwise. After the dropwise addition is complete, remove the ice bath and heat to reflux for 3-8h (preferably 6h), and the reaction solution is clarified. Cool down to below 50°C, generally at room temperature, add 1280g of anhydrous potassium carbonate, dropwise add ethyl haloacetate, 420g of ethyl chloroacetate in this example, heat up and reflux for 3-8h (preferably 5h). The reaction solution was cooled to room temperature, filtered with suction, the filtrate was concentrated, and ethanol was recovered. Add an organi...
Embodiment 2
[0017] The synthesis of quinuclidone (III) is realized through the following steps:
[0018] method one:
[0019] Take 100g intermediate 1-ethoxycarbonyl ethyl-4-piperidinecarboxylic acid ethyl ester (II), add 100mL toluene, stir evenly, add dropwise in the alkaline solvent, this embodiment is the reaction of 200mL toluene of 56g sodium ethylate In the solution, the reaction solution changed from clear to light red. After the dropwise addition, the temperature of the reaction system is about 80-90°C (preferably: 85°C). Under normal pressure conditions, the reaction solvent in the system was evaporated until the system temperature reached about 80-110° C., and the reaction was carried out for 6-10 hours. TLC / GC detected that the reaction of the raw materials was complete. Stop the reaction, cool down to room temperature, add dilute acid (such as dilute hydrochloric acid or dilute sulfuric acid), this example is 200mL dilute hydrochloric acid (2mol / L), stir for 0.5h, let stand...
Embodiment 3
[0027] The synthesis of quinucidine hydrochloride (V) is realized through the following steps:
[0028] 1) Method 1:
[0029] Take 1Kg of quinine (III) hydrochloride, add it to 2.9L of 2N sodium hydroxide solution several times under ice bath conditions, then add 65.6g of sodium carbonate solid, stir for 0.5h, and then use dichloro Extracted with methane (1.5L×3), combined the dichloromethane layers, dried over anhydrous sodium sulfate, concentrated and dried to obtain 670g of intermediate quinine ketone, with a yield of 87%. The above-mentioned quinine was slowly added to 1.3L 95% hydrazine hydrate in small amounts several times, then heated to reflux at 120°C overnight, cooled, and left at 0°C for 3h, crystals precipitated. After filtering, the crystals were rinsed three times with 800 mL of tert-butyl ether, and the filtered mother liquor was placed in a refrigerator to repeat the above operation, and combined to obtain 670 g of white crystals of 3-quinuclidine hydrazone. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com