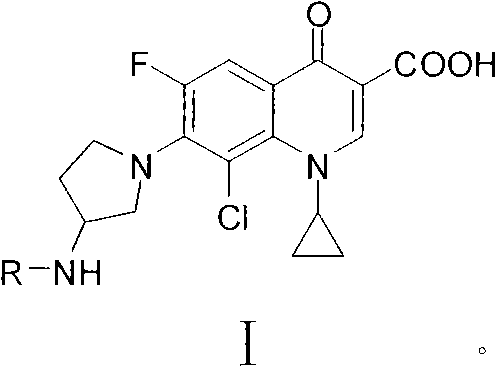
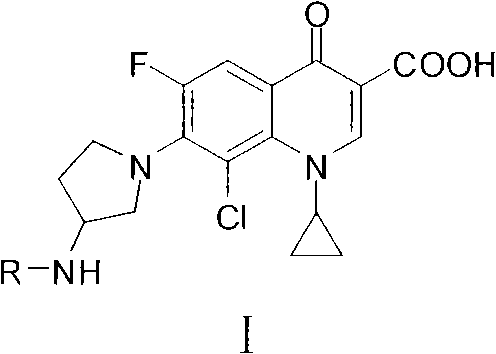
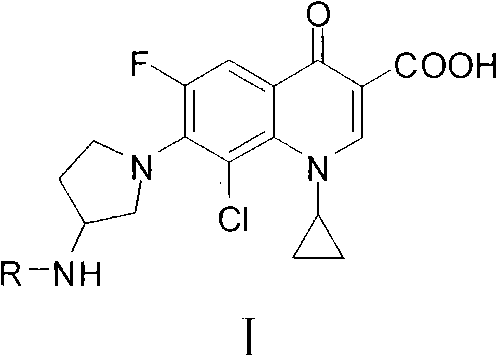
Clinafloxacin amino derivatives and application thereof
A derivative, star amino technology, applied in the field of chemistry and pharmacy, can solve problems such as solution instability, poor solubility, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1, the synthesis of some Clinfloxacin sulfonamido derivatives
[0061] Synthesis of compounds HHY-1~HHY-8: Add 0.8g (20mmol) of sodium hydroxide, 3.66g (10mmol) of clinfloxacin and 50mL of water into the reaction flask, stir to dissolve, then slowly add the corresponding The acetone solution of the reaction raw material (11-15 mmol) was added, and the temperature was raised to 40-80° C. to control the temperature and stir for reaction, and the reaction progress was monitored by thin-layer chromatography (TLC). After the reaction, adjust the pH of the reaction solution to 3-4 with 2mol / L hydrochloric acid solution, let it stand overnight at room temperature, filter it with suction, wash the filter cake with anhydrous methanol, and suspend it in anhydrous methanol, ethanol or isopropanol 60 -200mL, stir at room temperature for 2-4 hours, filter with suction, wash the filter cake with anhydrous methanol, and dry to obtain the corresponding target compound. The ...
Embodiment 2
[0093] Embodiment 2, the synthesis of some Clinfloxacin carbonyl amino derivatives
[0094] A, the synthesis of compound HHY-10~HHY-24
[0095] Add the corresponding reaction materials (24mmol), 3.24g (24mmol) of 1-hydroxybenzotriazole (HOBt) and 20mL of tetrahydrofuran (THF) into the reaction flask, stir to dissolve, then add dicyclohexyl carbonization under ice bath stirring Diimine (DCC) 4.94g (24mmol) and triethylamine (TEA) 2.02g (20mmol), after stirring in an ice bath for 30 minutes, add 7.32g (20mmol) of Clinfloxacin, and add an appropriate amount of THF (to facilitate stirring) , the temperature was raised to 20-45° C., the temperature was controlled and the reaction was stirred, and the reaction progress was monitored by TLC method. After the reaction, the reaction solution was left standing overnight at 4°C, filtered with suction, the filter cake was fully washed with dichloromethane (DCM), the washing liquid was combined with the filtrate, and the solvent was disti...
Embodiment 3
[0230] Embodiment 3, the agar diffusion antibacterial test of partial clinfloxacin amino derivatives
[0231] Melt common agar medium, cool to 55-60℃, add 1.0×10 6 CFU / mL test bacteria suspension (Staphylococcus aureus ATCC29213, Salmonella SR96-1 or Pseudomonas aeruginosa ATCC27853) 1.0mL, poured into a 90mm diameter petri dish, solidified and punched for later use. A phosphate buffer solution (100 mmol / L, pH 7.2) containing clinfloxacin amino derivatives was added to the above agar wells, and the amount of clinfloxacin amino derivatives added was 2 μg / well. After adding, culture at 37°C for 24 hours, and measure the diameter of the inhibition zone. The results are shown in Table 5. It can be seen that the amino derivatives of Clinfloxacin of the present invention have an inhibitory effect on Staphylococcus aureus ATCC29213, Salmonella SR96-1 and Pseudomonas aeruginosa ATCC27853, and the intensity of action of most derivatives is close to or superior to that of Staphylococcu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com