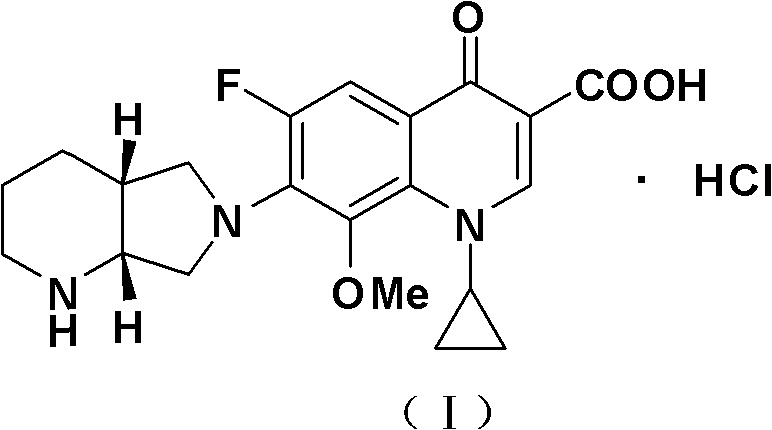
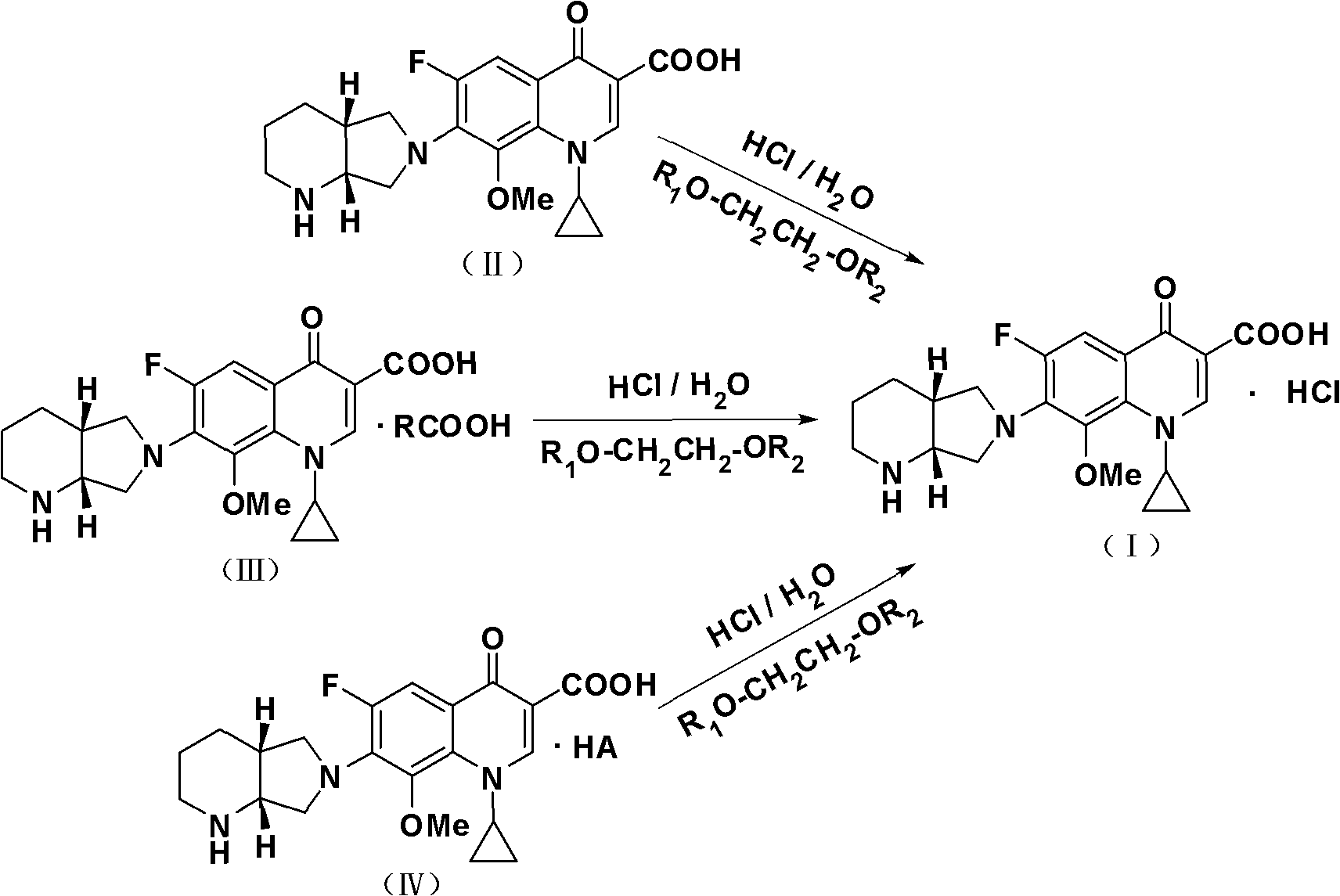
Process for crystallizing moxifloxacin hydrochloride
A technology of moxifloxacin hydrochloride and moxifloxacin, which is applied in the crystallization process field of moxifloxacin hydrochloride, can solve the problem of unfavorable quality stability of moxifloxacin hydrochloride, poor crystal form and quality of moxifloxacin hydrochloride, and affect the normal operation of wastewater treatment stations function and other issues, to achieve the effect of high product yield, good volatility, and reduced water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Put 30 grams of moxifloxacin malate (0.0560 mol), 25 grams of water and 30 grams of ethylene glycol mono-n-propyl ether into a 500 ml three-necked reaction flask in sequence, stir, heat to 75-80 °C, dissolve and add 0.2 gram of activated carbon, decolorize at 75-80°C for 30 minutes, filter while hot, and collect the filtrate. Heat the filtrate to 75-80°C, add 9 ml of concentrated hydrochloric acid dropwise to the filtrate until the system pH=1.5-2.5, keep stirring for 0.5 hours, fully analyze the crystallization at 75-80°C, and then start to drop 180 g of ethylene glycol mono For n-propyl ether, drop it within 1 hour, then start to lower the temperature, and keep warm and crystallize for 1.5 hours at 0~-2°C. Finished, suction filtered, rinsed the product with 35 grams of 90% ethanol, sucked dry, and vacuum-dried for 6 to 7 hours at 60 to 65° C. to obtain 22.7 grams of moxifloxacin hydrochloride (I), with a molar yield of 92.6%.
Embodiment 2
[0034] Put 30 grams of moxifloxacin tartrate (0.0544 moles), 30 grams of water and 25 grams of ethylene glycol dimonoether into a 500 ml three-necked reaction flask in sequence, stir, heat to 75-80 ° C, add 0.2 grams of activated carbon after dissolving , decolorized at 75-80°C for 30 minutes, filtered while hot, and collected the filtrate. Heat the filtrate to 75-80°C, add 9 ml of concentrated hydrochloric acid dropwise to the filtrate until the pH of the system is 1.5-2.5, keep stirring for 0.5 hours, fully analyze the crystallization at 75-80°C, and then start to add 190 g of ethylene glycol di Monoether, drop it within 1 hour, then start to lower the temperature, and keep warm and crystallize for 1.5 hours at 0~-2°C. Finish, suction filtration, be 10% ethylene glycol dimethyl ether solution rinsing product with 35 grams of water content, suck dry, vacuum-dry 5~6 hours at 50~55 ℃, obtain 22.9 grams of moxifloxacin hydrochloride (I ), the molar yield is 96.1%.
Embodiment 3
[0036] Put 30 grams of moxifloxacin mandelate (0.0542 mol), 25 grams of water and 27 grams of ethylene glycol mono-n-propyl ether into a 500 ml three-necked reaction flask in turn, stir, heat to 75-80 °C, dissolve and add 0.2 gram of activated carbon, decolorize at 75-80°C for 30 minutes, filter while hot, and collect the filtrate. Heat the filtrate to 75-80°C, add 9 ml of concentrated hydrochloric acid dropwise to the filtrate until the system pH=1.5-2.5, keep stirring for 0.5 hours, fully analyze the crystallization at 75-80°C, and then start to drop 190 g of ethylene glycol mono For n-propyl ether, drop it within 1 hour, then start to lower the temperature, and keep warm and crystallize for 1.5 hours at 0~-2°C. Finished, suction filtered, rinsed the product with 35 grams of 90% alcohol, drained, and dried in vacuum at 60 to 65° C. for 6 to 7 hours to obtain 22.1 grams of moxifloxacin hydrochloride (I), with a molar yield of 93.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com