Method for producing N-(N-butyl)thiophosphoric triamide in channelization manner and special equipment
An n-butyl thiophosphoric triamide, pipelined technology, applied in chemical instruments and methods, chemical methods for reacting liquids and gaseous media, organic chemistry, etc. The acid agent triethylamine has an unpleasant odor and high production conditions, so as to overcome the uneven local concentration, easy control of reaction conditions and stable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
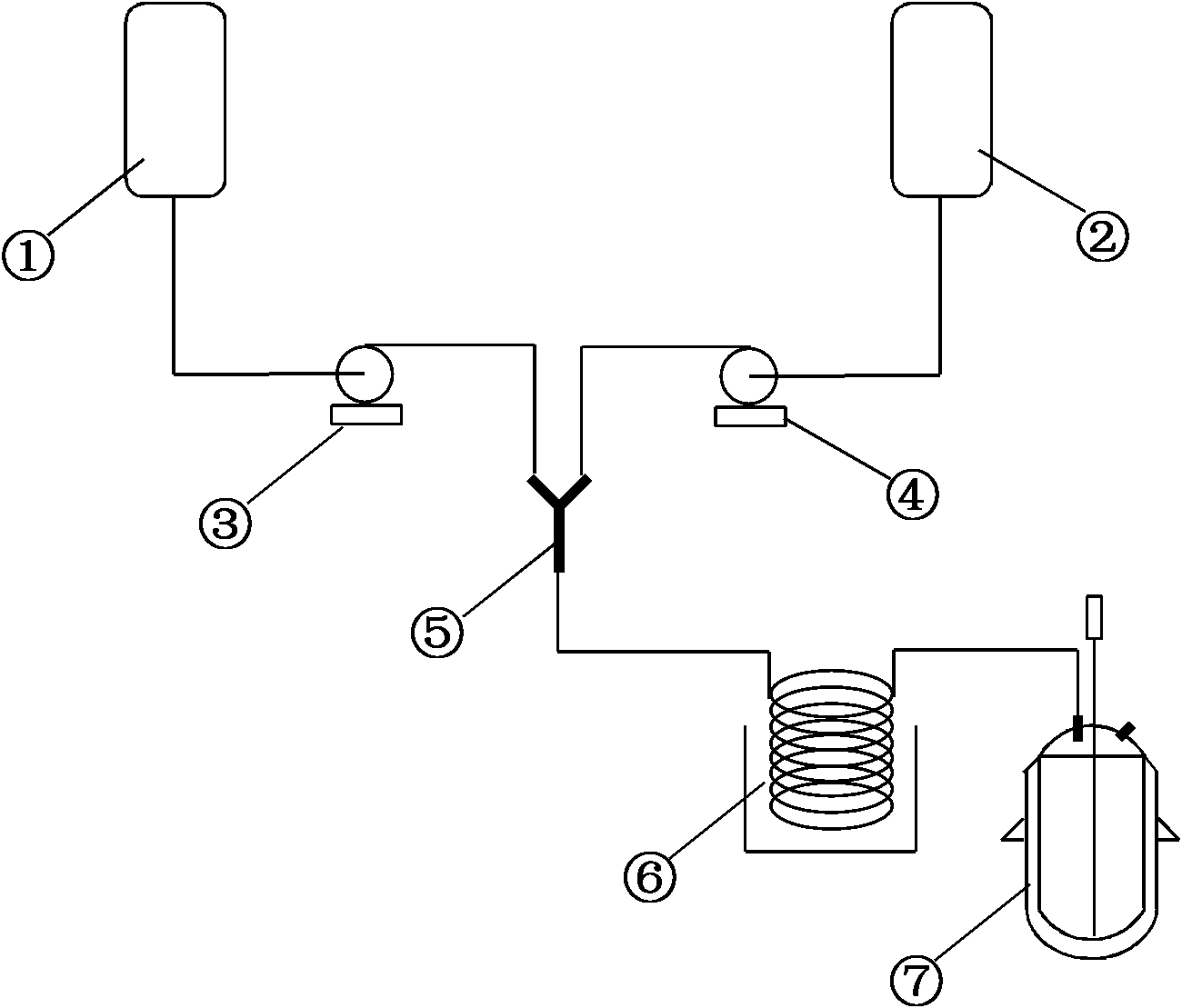
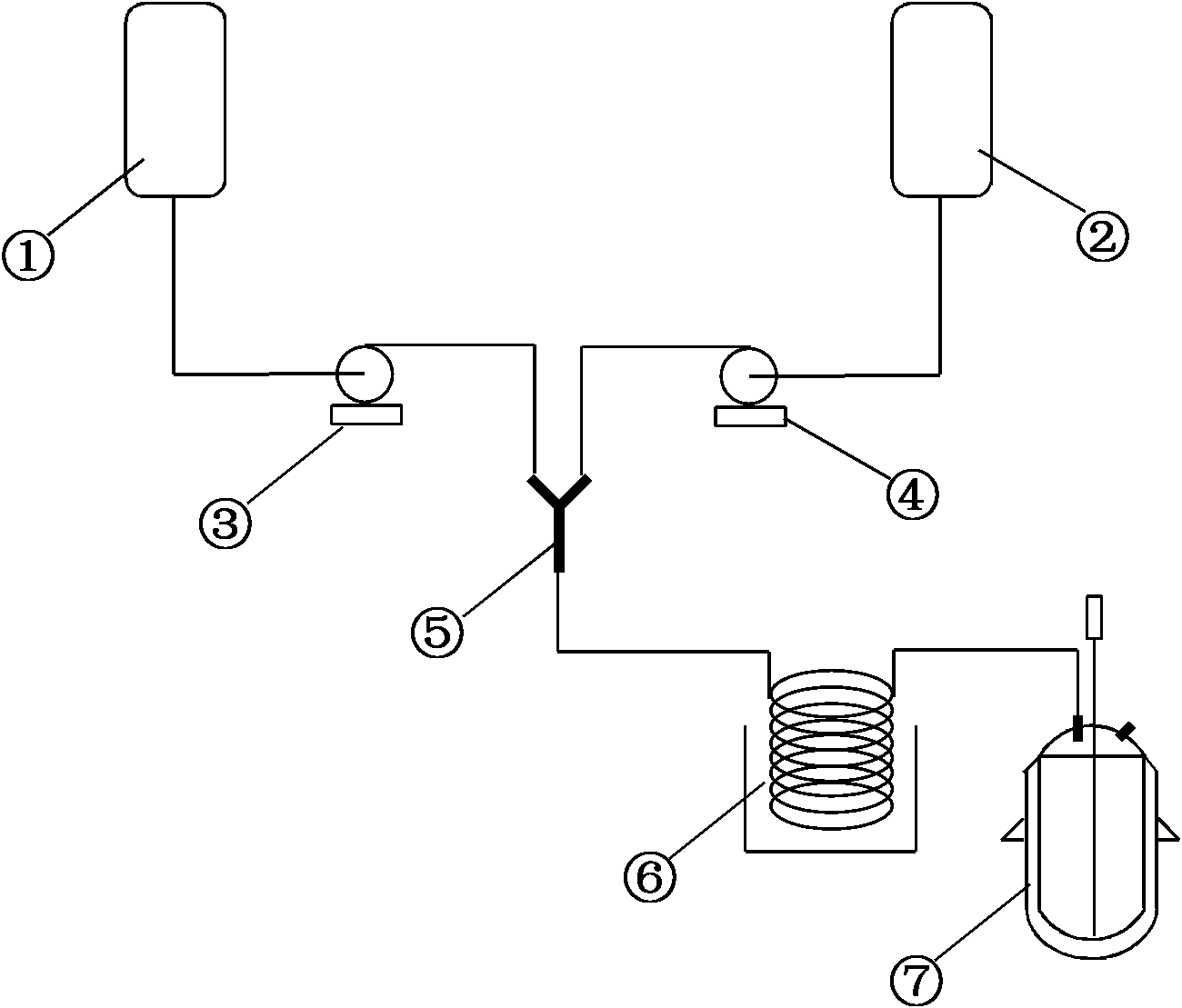
[0026] 3kg (17.7mol) of phosphorus trichloride and 2.14kg (29.2mol) of n-butylamine were transported to the Y-type jet mixer respectively, and after mixing, they entered the tubular reactor (single tube, tube length 10 meters, tube diameter 8 mm) , the reaction temperature is controlled at 10°C, and the residence time is 20s. After the reaction is completed, the reaction liquid directly enters the amination reactor with 7.5kg of dichloromethane added in advance, and the reaction temperature is controlled at 25°C under normal pressure for 3 hours. , add 4kg of water directly, stir for 1h, let stand to separate layers, wherein about 75% of the solvent is recovered by distillation of the organic layer under reduced pressure, and the residue is cooled to -5~0°C to crystallize to obtain 2.46kg of pure n-butylthiophosphoric triamide , as white crystals, with a melting point of 57-58°C, a yield of 83.1%, and a purity of 97.8%.
Embodiment 2
[0028] 3kg (17.7mol) of phosphorus trichloride and 2.14kg (29.2mol) of n-butylamine were transported to the Y-shaped jet mixer respectively, and after mixing, they entered the tubular reactor (single tube, tube length 5 meters, tube diameter 3 mm) , the reaction temperature is controlled at 35°C, and the residence time is 10s. After the reaction, the reaction solution is directly passed into the amination reactor with 6 kg of dichloromethane added in advance, and the cooling medium is passed through the jacket of the amination reactor. Continuously react with ammonia for 3 hours. After the stirring reaction is complete, directly add 4 kg of water, stir for 1 hour, and stand to separate layers. After the organic layer is distilled under reduced pressure to recover the solvent, the residue is added to 1.5 L of mixed solvent (dichloromethane: petroleum ether = 1 : 1) cooling to -5~0° C. to crystallize to obtain 2.43 kg of pure n-butylthiophosphoric triamide as white crystals with ...
Embodiment 3
[0030]3kg (17.7mol) of phosphorus trichloride and 2.14kg (29.2mol) of n-butylamine were transported to the Y-shaped jet mixer respectively, and after mixing, they entered the tubular reactor (single tube, 15 meters in length and 5 mm in diameter) , the reaction temperature is controlled at 15°C, and the residence time is 60s. After the reaction, the reaction solution is directly passed into the amination reactor with 12kg of dichloromethane added in advance, and the cooling medium is passed through the jacket of the amination reactor. Continuously react with ammonia for 3 hours. After the stirring reaction is complete, directly add 4kg of water, stir for 1 hour, and let stand to separate layers. After the organic layer is distilled under reduced pressure to recover about 75% of the solvent, the residue is cooled to -5~0°C to crystallize to obtain n-butyl 2.6 kg of pure thiophosphoric triamide is a white crystal with a melting point of 57-58°C, a yield of 87.8%, and a purity of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com