Transdermal administration kit
A transdermal drug delivery and kit technology, applied in the field of transdermal drug delivery kits, can solve the problem of not being able to maintain basal insulin through transdermal drug delivery, and achieve the effect of maintaining the level, cheap price, and slowly increasing the concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
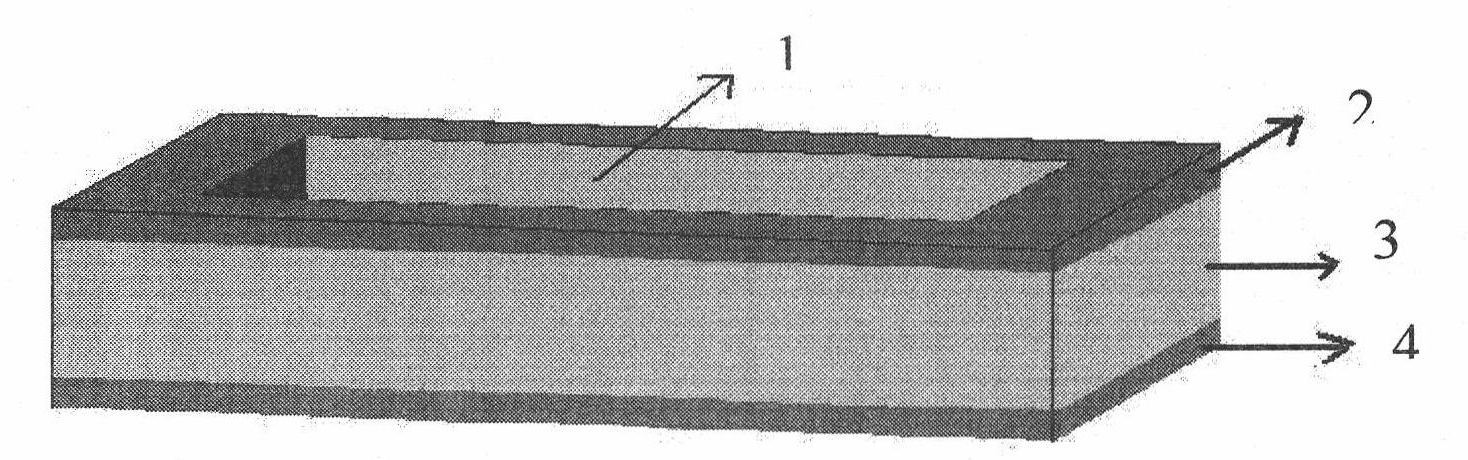
[0041] This example is used to illustrate the preparation method of the transdermal drug delivery patch of the transdermal drug delivery kit provided by the present invention.
[0042] Take 40mg of Carbomer 980 powder and add 100IU / ml of Novolin Insulin (Novo Nordisk's recombinant human insulin) 0.4ml (equivalent to 1.4 mg of recombinant human insulin, at this time the weight ratio of drug support body to recombinant human insulin is 28.5: 1), after mixing evenly, spread on an area of At the center of the non-woven fabric (substrate) of 1.6cm×2.6cm (length×width), a rectangular drug holder and recombinant human insulin coating of 1cm×2cm (length×width) is formed, with a thickness of about 2mm, and A pressure-sensitive adhesive about 3mm wide is pasted on the edge of the non-woven fabric. The transdermal drug delivery patch used in the transdermal drug delivery kit of the present invention is obtained.
Embodiment 2
[0044] This example is used to illustrate the preparation method of the transdermal drug delivery patch of the transdermal drug delivery kit provided by the present invention.
[0045]Take 50g of pre-polymerized polydimethylsiloxane (Dow Corning, silicone elastomer, batch number: 0005002533) and add 0.25g of crosslinking agent (Dow Corning, silicone elastomer curing agent, batch number: 0005002533), mix well and pour it on a glass plate , so that it spreads evenly, with a thickness of 3mm, and a release agent is pre-added on the glass plate (Dow Corning silicon-based release agent, so that the made silicone rubber patch can be easily removed from the glass plate without adhesion); then Put it in a 50°C oven for 2 hours and take it out, peel off the polymerized silicone rubber, and cut the obtained silicone rubber film into a cuboid with a size of 2.6cm×1.6cm×2mm (length×width×height) according to the needs of administration , on two opposite surfaces of the silicon rubber memb...
Embodiment 3
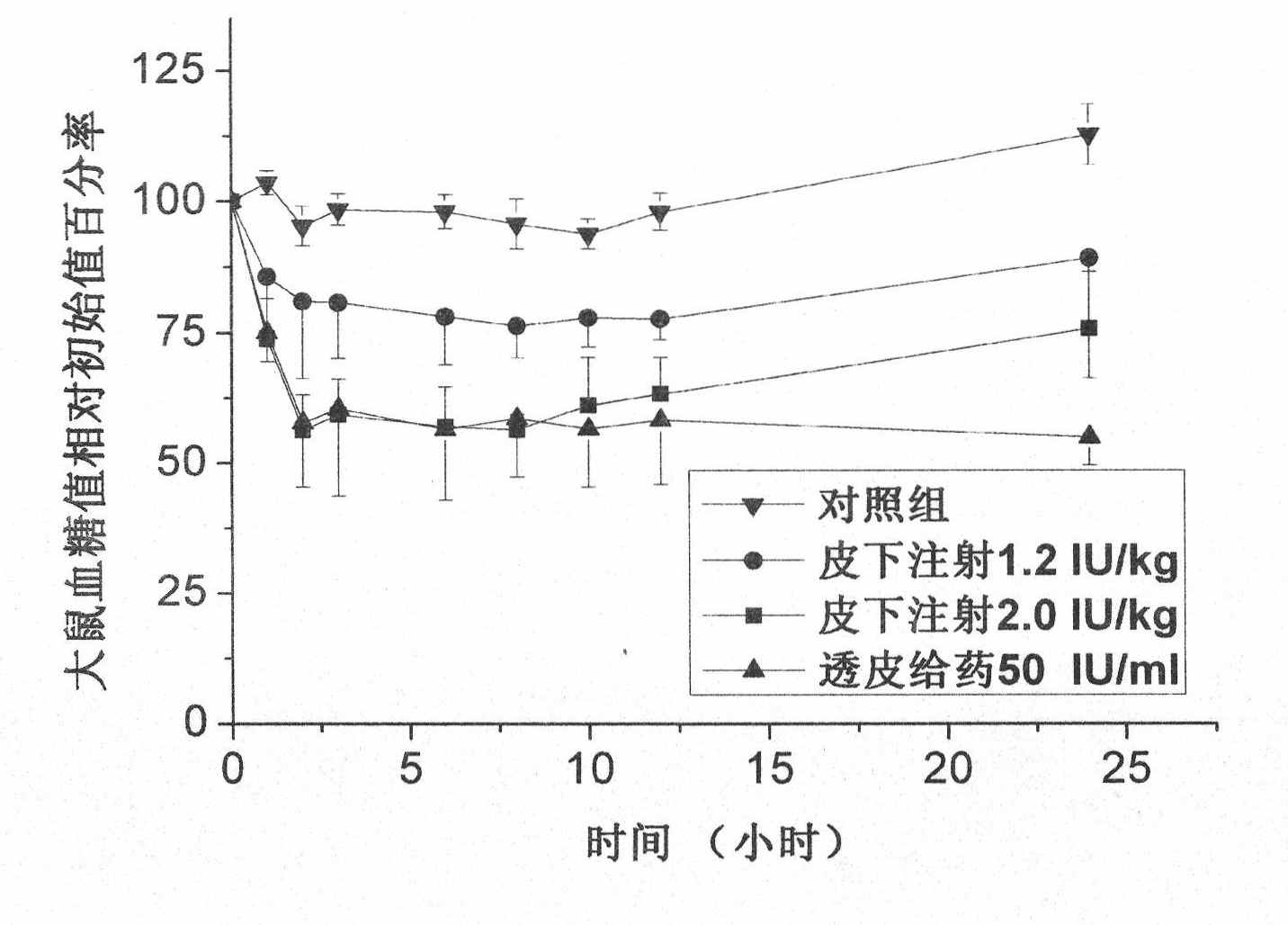
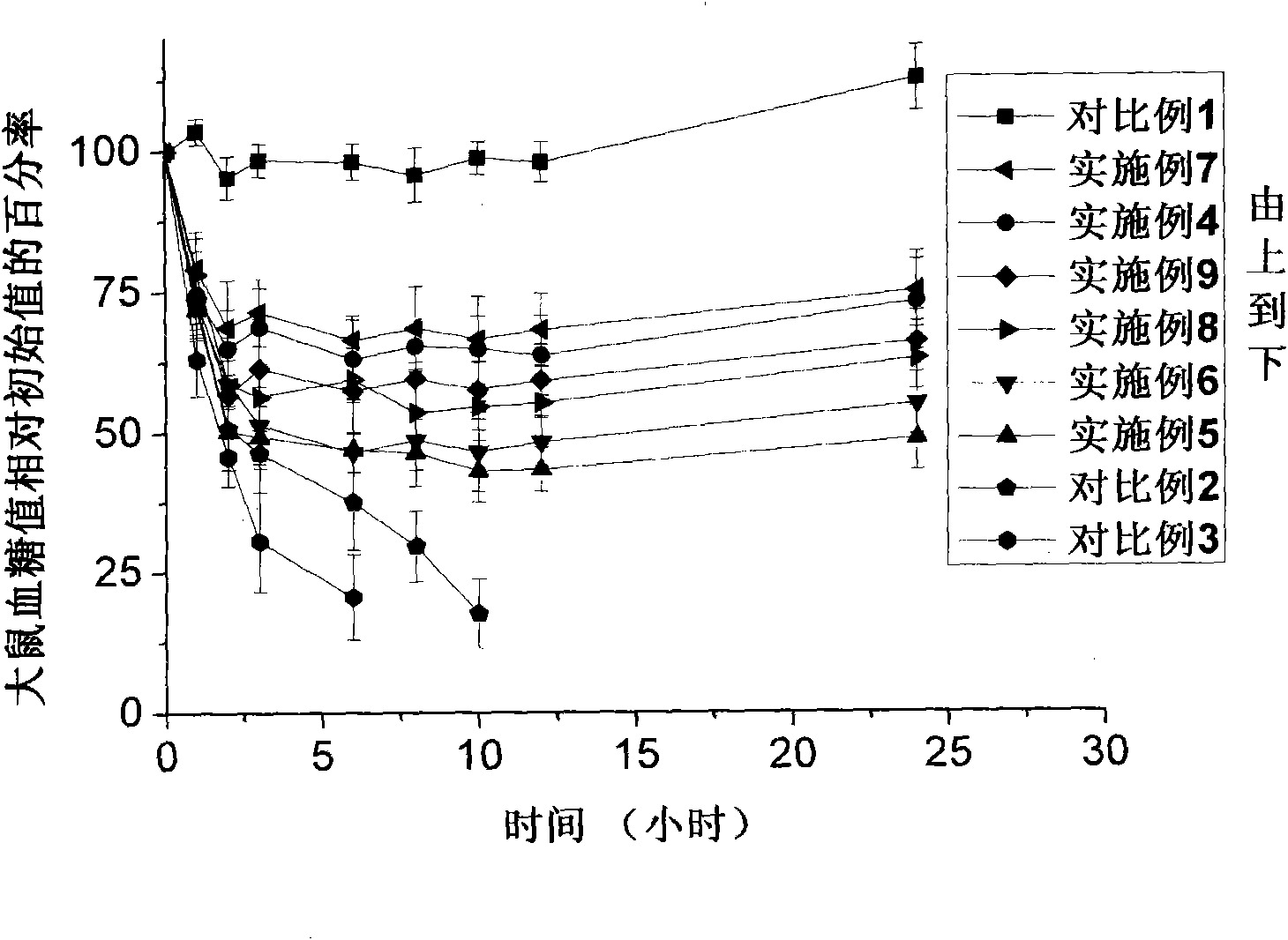
[0048] This embodiment is used to illustrate the usage method of the transdermal drug delivery kit provided by the present invention.
[0049] Adopt successfully induced diabetic rats as a model, remove the hair of its abdomen after ether anesthesia, and clean it with normal saline, adopt the microneedle array (eight-sided conical microneedle, microneedle) in the transdermal drug delivery kit of the present invention The height is 100 μm, the density is 484 needles / square centimeter, the diameter of the needle tip is 1 μm, the width of the needle bottom is 100 μm, and the area is 1cm 2 , Suzhou Nasheng Microelectronics Co., Ltd.) and the microneedle propeller with a frequency of 6000-20000 times / min of longitudinal vibration act on the depilated part of the rat for 10 seconds, and then act on the adjacent part of the first acting position for another 10 seconds , the total area of microneedle action is 1×2cm 2 . The transdermal patch is prepared according to the method of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com