Preparation method of imatinib mesylate
The technology of imatinib mesylate and benzoic acid dihydrochloride is applied in the field of preparation of imatinib mesylate, and can solve the problems of difficulty in removing reaction by-products, long total reaction time, many reaction steps, and the like, Achieve the effects of being beneficial to environmental protection, stable and feasible process, and moderate reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Implementation example 1: the first preparation method of imatinib mesylate
[0091] (1) Take Intermediate I (50.3g, 0.181mol), suspend it in 250ml of dimethylformamide (DMF), and add Intermediate II (75.2g, 0.245mol), and N-hydroxy Succinimide (1.04 g, 0.009 mol) was added to a solution of dicyclohexylcarbodiimide (39.2 g, 0.190 mol) in DMF within 10 minutes with stirring at room temperature. The reaction was stirred at 20°C for 17 hours, and the reaction was monitored by HPLC. After the reaction was completed, it was filtered, and the product was dried under reduced pressure at 60°C for 6 hours to obtain 60.1 g of the product as a light yellow solid.
[0092] (2) Dissolve 60 g of the light yellow solid obtained in step (1) in 240 ml of water, add 0.5% activated carbon, heat to 60° C. in a water bath, stir for 30 minutes, and filter. The pH of the filtrate was adjusted to 10.2 with aqueous ammonia, and the precipitate was filtered and dried in vacuo to obtain 49.8 g o...
Embodiment 2
[0098] Implementation example 2: the second preparation method of imatinib mesylate
[0099] (1) Take intermediate II (2350g, 7.65mol), add 20L of thionyl chloride under constant stirring, heat to boiling, slowly add 27ml of DMF dropwise, keep boiling for 2 hours, recover dichloromethane under reduced pressure For sulfone, appropriate amount of toluene was added several times and evaporated to dryness under reduced pressure to remove excess thionyl chloride, and dried under vacuum at 60°C.
[0100] (2) Take intermediate I (1450g, 5.23mol), add 7.2L of DMF, stir to dissolve, add the acid chloride generated by the above reaction, heat to 70°C, add 1L of pyridine, stir for about 15 hours, and monitor the progress of the reaction by HPLC After the reaction is completed, cool to room temperature, stir for 2 hours, filter, and dry the product under reduced pressure at 60°C.
[0101] (3) Take the imatinib hydrochloride obtained by the above reaction, add 7.5L of water, stir to disso...
Embodiment 3
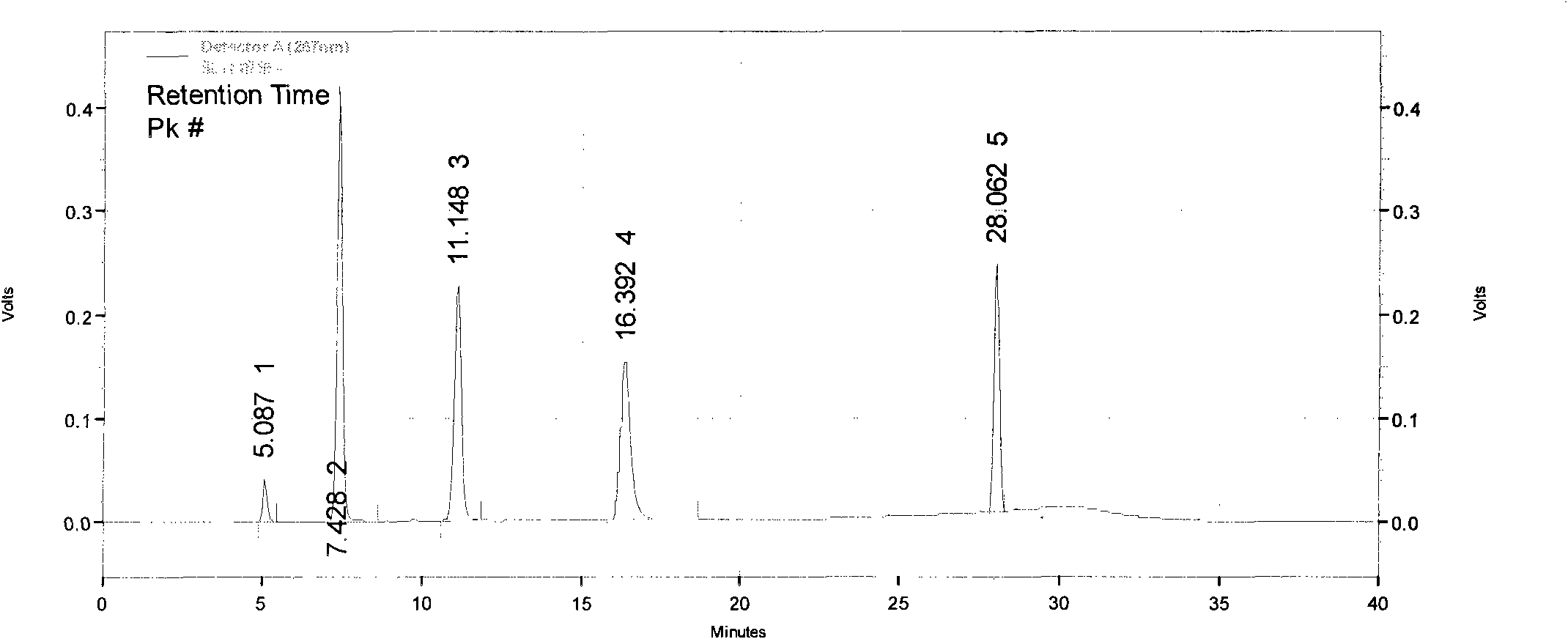
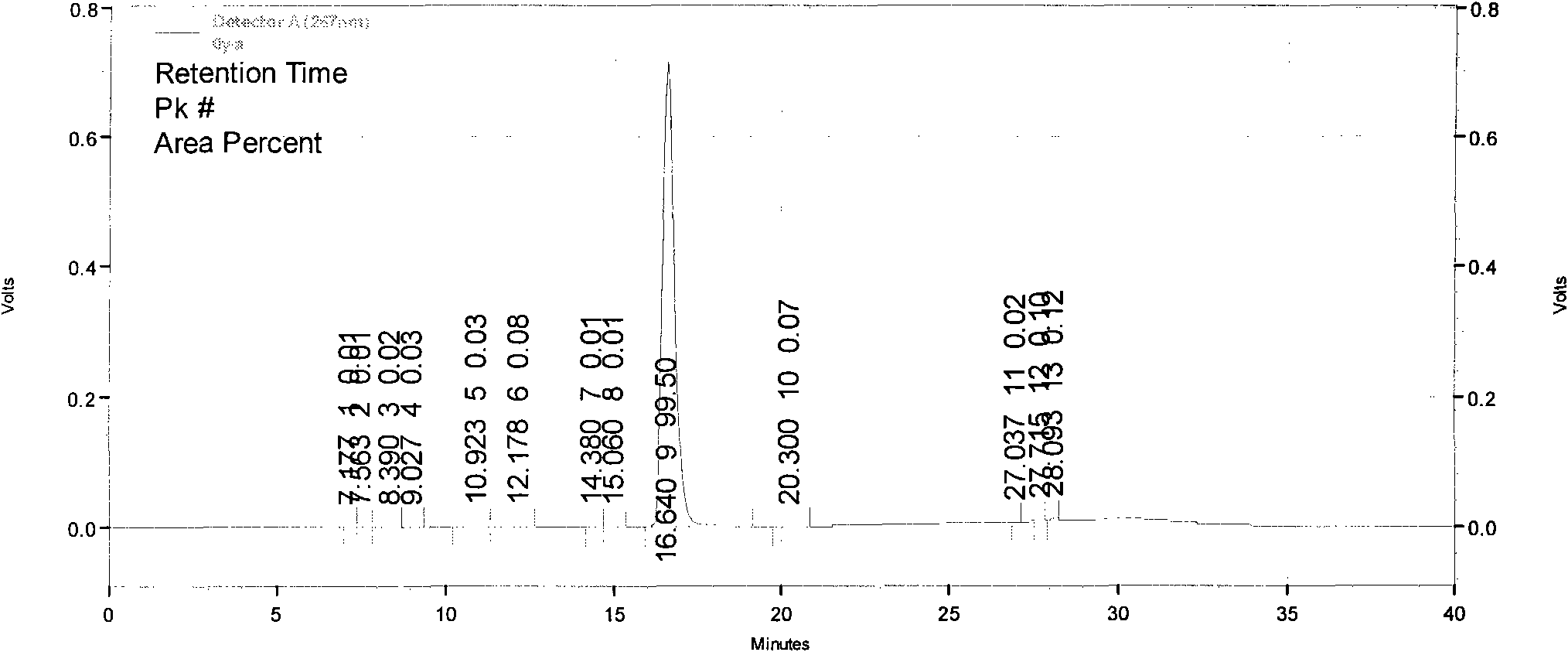
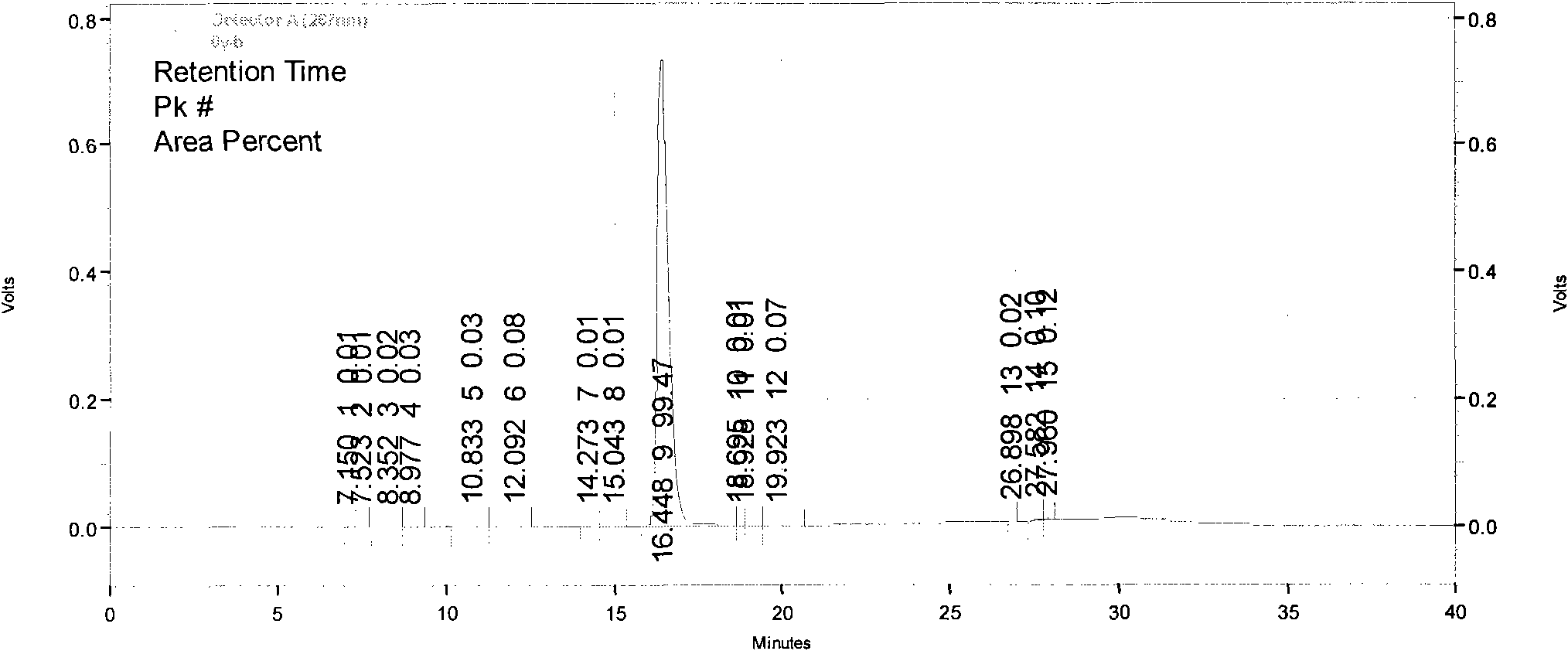
[0106] Implementation example 3: HPLC purity detection of imatinib mesylate
[0107] Chromatographic conditions:
[0108] Chromatographic column: Venusil C18 (250×4.6mm, 5μm)
[0109] Mobile phase: sodium octane sulfonate solution (take 7.5 g of sodium octane sulfonate, add 1000 ml of water to dissolve, adjust the pH value to 2.5 with 10% phosphoric acid) + methanol-acetonitrile (1:1), gradient elution, the gradient conditions are the same as above .
[0110] Column temperature: room temperature
[0111] Flow rate: 1.0ml / min
[0112] Detection wavelength: 267nm
[0113] Injection volume: 10μl
[0114] Preparation of mixed resolution solution: Take intermediate I, intermediate II, imatinib mesylate, appropriate amount of impurity A, and impurity B, and add methanol to make a solution containing 0.1 mg per 1 ml as a resolution solution.
[0115] Precisely measure 10 μl, inject it into the chromatograph, and record the chromatogram. The results show that the resolution of eac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com