Preparation method of tolvaptan
A technology of tolvaptan and intermediates, applied in the field of chemical pharmacy, can solve problems such as unfavorable workshop safety operation, flammability and explosion of anhydrous ether, serious environmental pollution, etc., to achieve environmental protection and reduce labor protection requirements , The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
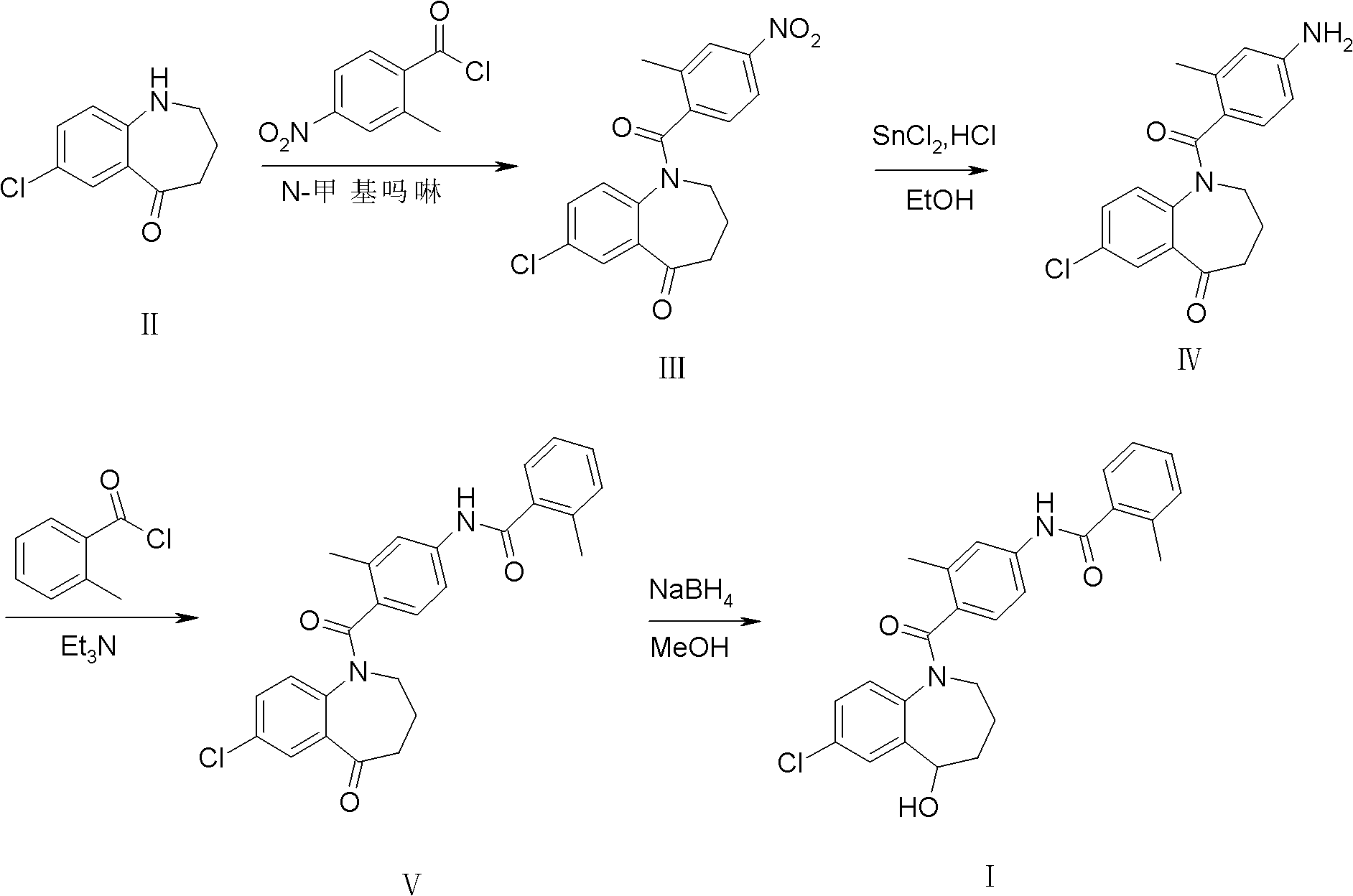
[0027] Embodiment 1: the synthesis of intermediate III
[0028] 2-Methyl-4-nitrobenzoic acid (purchased from Tianjin Alpha Aisha Chemical Co., Ltd., purity>99%, 25g, 0.14mol) was added to a 250ml reaction bottle, and reacted with thionyl chloride for 3h under reflux conditions , thionyl chloride was distilled off under reduced pressure to obtain 2-methyl-4-nitrobenzoyl chloride (26.8 g, light yellow oily liquid), which was directly used in the next step without purification.
[0029] Intermediate II (20 g, 0.1 mol) and 2-methyl-4-nitrobenzoyl chloride (22.4 g, 0.11 mol) were added to a 250 ml reaction flask. Add dichloromethane (50ml), cool in an ice bath to 0-5°C and stir until dissolved, slowly add N-methylmorpholine (11.2g, 0.11mol) dropwise, stir for a while after dropping, and react at room temperature for 4h. TLC [developing solvent: ethyl acetate-petroleum ether (1:1), the same below] shows that after the reaction is complete, add saturated aqueous sodium bicarbonate (...
Embodiment 2
[0030] Embodiment 2: the synthesis of intermediate IV
[0031] Intermediate III (10g, 28mmol) was added to a 250ml reaction flask, concentrated hydrochloric acid (40ml) and ethanol (50ml) were added, stirred, and an ethanol solution (40ml) of stannous chloride (20g, 88mmol) was slowly added dropwise. After dropping, react at room temperature for 5h. After TLC showed that the reaction was complete, about 70 ml of ethanol was distilled off under reduced pressure, and the residue was cooled at -10°C-0°C overnight. After filtering, the filter cake was washed with water and poured into water (40ml). Add 20% aqueous sodium hydroxide solution (about 60ml) to adjust to pH 9. Filter and recrystallize with absolute ethanol to obtain light yellow powder solid IV (6.3 g, 68.7%), mp 190.4-191.1°C. Purity 97.2% (HPLC normalization method).
Embodiment 3
[0032] Embodiment 3: the synthesis of intermediate V
[0033] Intermediate IV (5g, 15mmol) and triethylamine (2.3g, 23mmol) were sequentially added to a 100ml reaction flask, dichloromethane (30ml) was added, and stirred until completely dissolved. Add o-toluoyl chloride (2.8 g, 18 mmol) dropwise, and react at room temperature for 1 h after dropping. TLC showed that the reaction was complete and then poured into ice water (about 40ml), extracted with dichloromethane (20ml×3), combined the organic phases, and washed successively with 5% hydrochloric acid (25ml×3) and saturated sodium chloride solution (25ml×3 ), washed with anhydrous sodium sulfate, and filtered. The solvent (about 50ml) was recovered from the filtrate under reduced pressure, and the residue was recrystallized from anhydrous methanol-petroleum ether (2:1) to obtain white crystal intermediate V (6.2g, 90.9%), mp 121.1-123.6°C. Purity 98.6% (HPLC normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com