Rare earth ternary complex as well as preparation method and application thereof
A technology of ternary complexes and rare earths, which is applied in the direction of botanical equipment and methods, applications, and compounds containing elements of Group 3/13 of the periodic table. The effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of ligand 2-pyridinecarbaldehyde thiosemicarbazone Schiff base
[0036] Dissolve 0.05mol of 2-pyridinecarbaldehyde in 50mL of absolute ethanol, place it in a 150mL round-bottomed flask, and slowly add 4.557g (0.05mol) of thiosemicarbazide in absolute ethanol solution dropwise under stirring. Reflux at 80°C for 4 hours. After the reaction, cool and a crystalline white solid precipitates out. Filter and wash with ethanol three times. The obtained solid is dried in a vacuum oven for 4 to 6 hours to obtain the pure product. (yield: 89%; melting point: 197.2~197.3)
[0037]
[0038] (2) Preparation of rare earth ternary complexes
[0039] Weigh 0.18g (1mmol) 2-pyridineformaldehyde thiosemicarbazone Schiff base and dissolve it in 5mL of hot absolute ethanol solution. 3 ) 3 ·6H 2 The methanol solution of O was slowly added dropwise to the above solution, stirred and refluxed for 4 hours, and then slowly added dropwise an absolute ethanol solution contain...
Embodiment 2~12
[0042] The difference between the following examples and Example 1 is that the Ln ligands and Hq ligands used in the preparation of rare earth ternary complexes are different, as long as the 8-hydroxyquinoline derivatives of the same molar number are substituted for 8- Hydroxyquinoline, see Table 1 for details. The rest of the content is the same as described in Example 1.
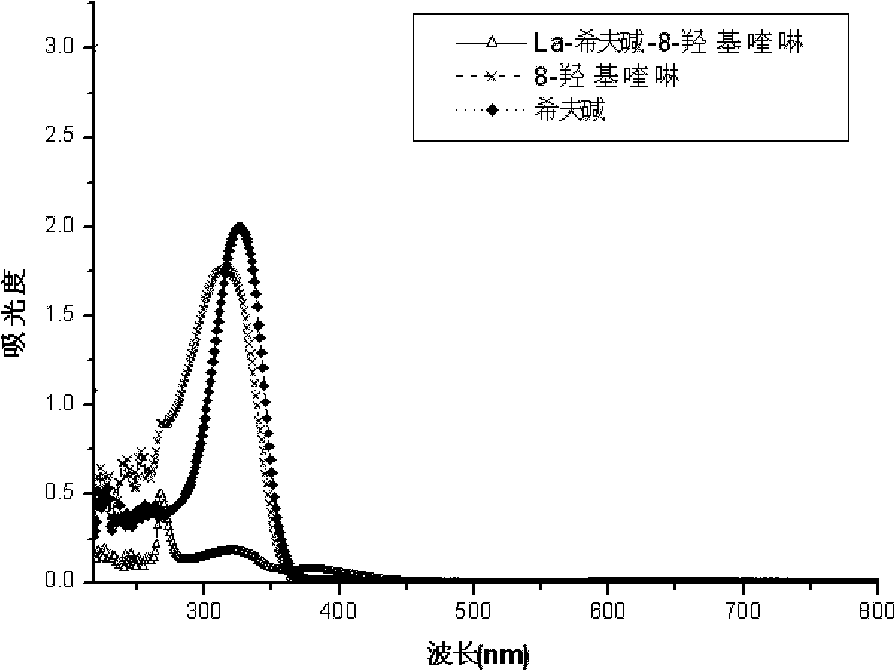
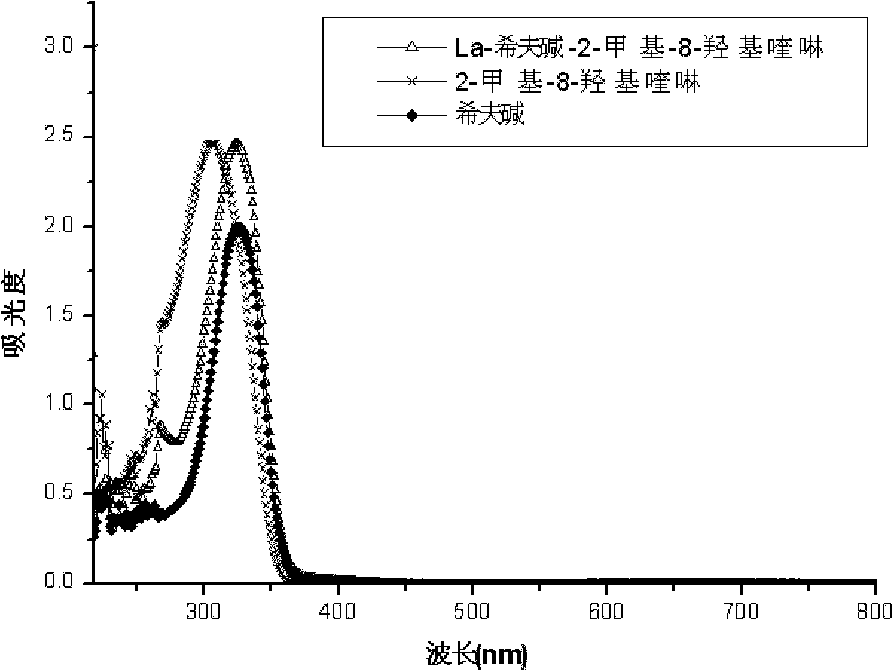
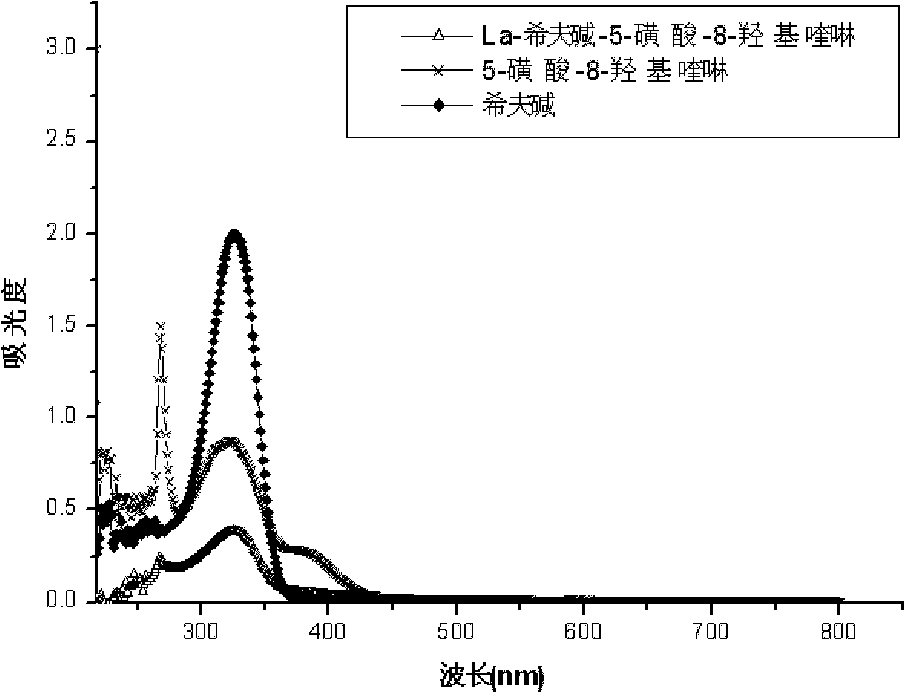
[0043] Figure 1-Figure 3 For the ultraviolet data that the prepared rare earth ternary complexes and their ligands of Example 1 are measured in DMF solution, it can be found that the ternary complexes of the same ligands have similar spectra from the ultraviolet spectrograms, and the ternary complexes of the complexes The spectra are distinct from those of the ligands. The Schiff base ligand has two absorption peaks at 260nm and 324nm respectively. After the complex is formed, the two absorption peaks have red-shifted. Distortion, the delocalized conjugation effect is weakened, and the CH=N double bond...
Embodiment 13
[0051] media diffusion method
[0052] (1) Principle: The antibacterial agent is continuously dissolved and diffused through agar to form different concentration gradients to show its antibacterial effect.
[0053] (2) Operation steps:
[0054] ① Preparation of antibacterial agent: Dissolve the rare earth complex in DMF, add a small amount of Tween 80 to solubilize and emulsify, and prepare a 0.005mol / L suspension concentrate, 8-hydroxyquinoline, 2-methyl-8-hydroxyquinoline, 5-sulfonic acid-8-hydroxyquinoline, 5-nitro-8-hydroxyquinoline, Ln(NO 3 ) 3 ·6H 2 O and Schiff's base were prepared into 0.005mol / L solutions respectively.
[0055] ②Preparation of antibacterial sheet: take a sterile and dry filter paper sheet (pore diameter 5mm), then wrap the filter paper sheet with double-layer newspaper and place it in a sterilizer for sterilization. After the sterilization is completed, put it in an incubator (37 ℃) after drying.
[0056] ③Inoculation of test bacteria: Dip with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com