Quinolone derivatives and application thereof in organic light-emitting devices (OLEDs)
A technology of organic groups and compounds, applied in the field of organic electroluminescent materials, can solve the problem of low fluorescence quantum efficiency and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
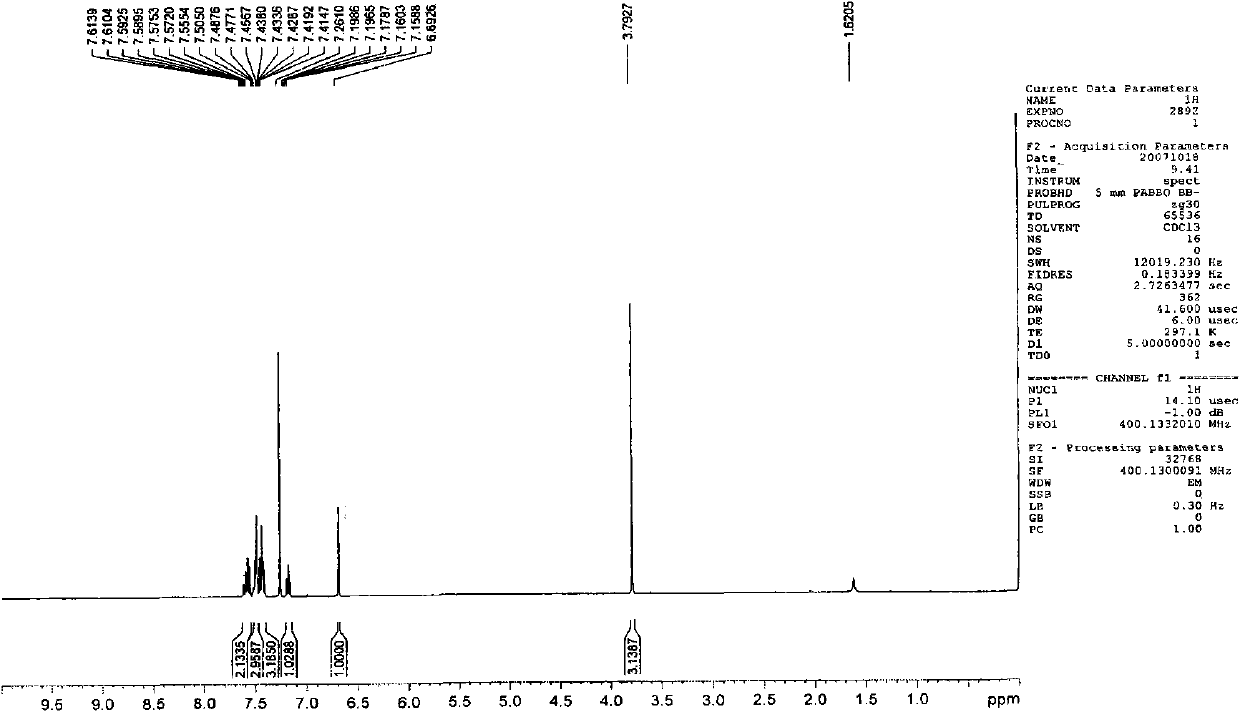
[0075] Embodiment 1,1-methyl-3, the preparation of 4-diphenylquinolin-2 (1H)-ketone (Q1)
[0076]
[0077] The first step: take 1-methyl-4-phenylquinolin-2(1H)-one with a molar ratio of 1:1, and N-bromosuccinimide as raw materials, and dissolve the above-mentioned raw materials in N, N-dimethylformamide was reacted with stirring at room temperature for 10 hours, and a large amount of solid was precipitated by adding water, which was washed and dried to obtain the corresponding bromide with a yield of 80%.
[0078] The second step: under the protection of nitrogen, the bromide obtained in the first step and phenylboronic acid (molar ratio is 1: 1) are dropped into two-necked flasks, and a catalytic amount of tetrakis (triphenyl) phosphine palladium) and mixed solvent ( Sodium carbonate solution (2mol / L):toluene:ethanol=1:4:4), keep the temperature at 90-100°C, stir for 24 hours, extract with ether, and the product is subjected to column chromatography (eluent is petroleum et...
Embodiment 2
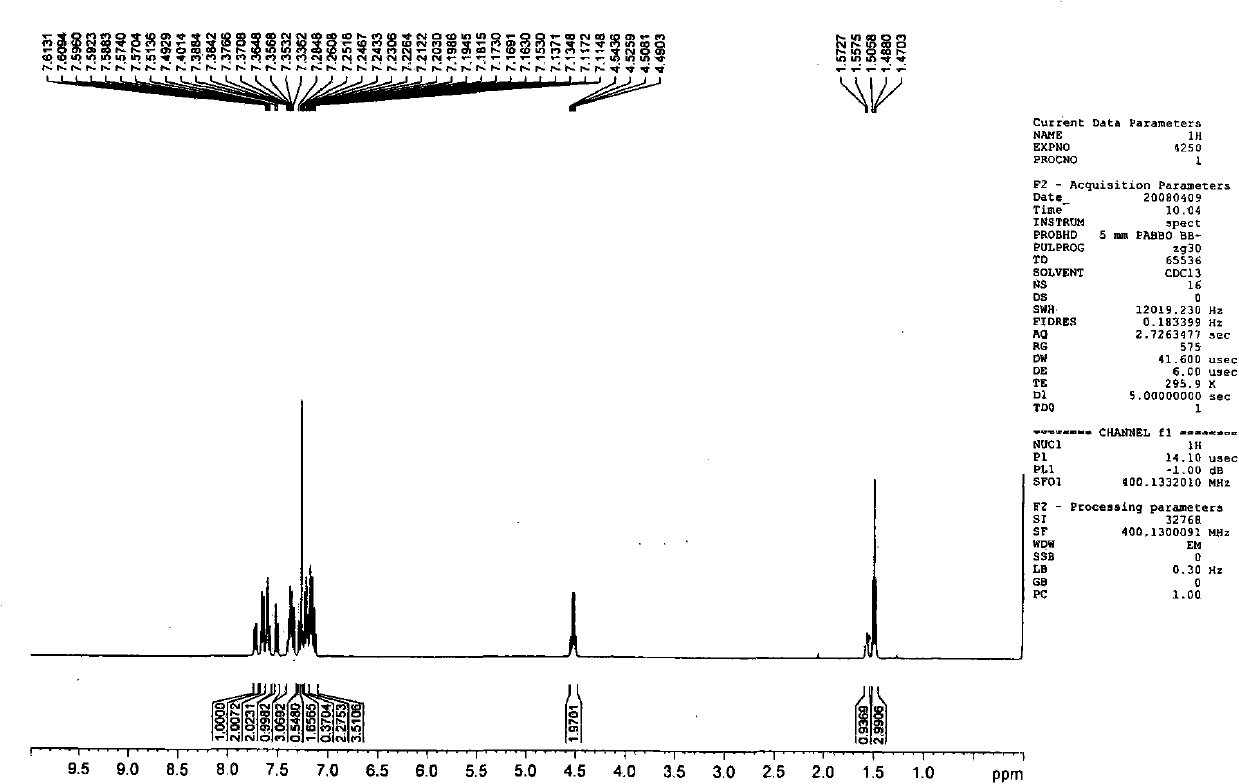
[0090] Embodiment 2, the preparation of 1-methyl-3-(2-naphthyl)-4-phenylquinolin-2(1H)-one (Q2)
[0091]
[0092] The first step: take 1-ethyl-4-phenylquinolin-2(1H)-one with a molar ratio of 1:1, and N-bromosuccinimide as raw materials, dissolve the above raw materials in N, N-dimethylformamide was stirred at room temperature for 10 hours, and a large amount of solid was precipitated by adding water. The solid was washed and dried to obtain the corresponding bromide with a yield of 80%.
[0093] The second step: under the protection of nitrogen, put the product obtained in the first step and 2-naphthylboronic acid (the molar ratio is 1:1) into a two-neck flask, add a catalytic amount of tetrakis (triphenyl) phosphine palladium) and a mixed solvent (sodium carbonate solution (2mol / L):toluene:ethanol=1:4:4), keep the temperature at 90-100°C, stir for 24 hours, extract with ether, and the product is subjected to column chromatography (eluent is petroleum ether / ethyl acetate ...
Embodiment 3
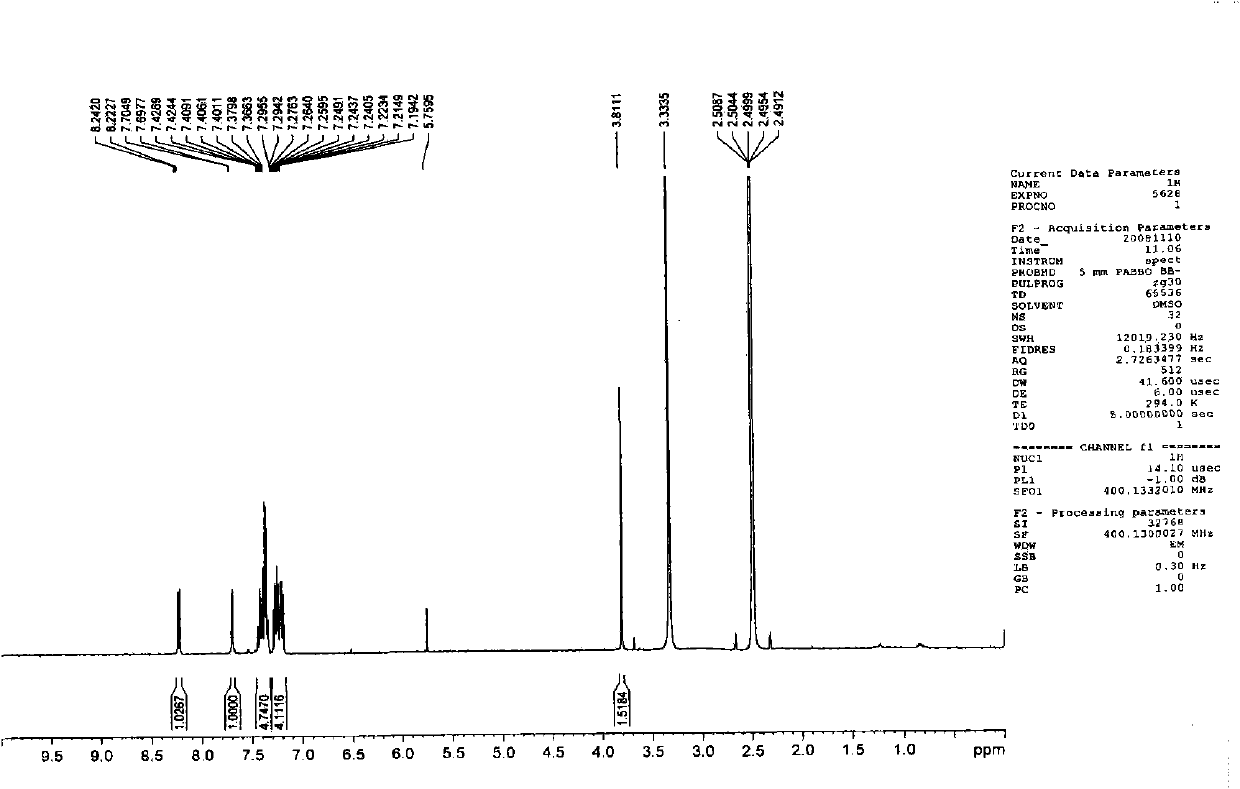
[0103] Embodiment 3, the preparation of 1-methyl-3-(4-(9-carbazolyl)phenyl)-4-phenylquinolin-2(1H)-one (Q3)
[0104]
[0105] The first step: take 1-methyl-4-phenylquinolin-2(1H)-one with a molar ratio of 1:1, and N-bromosuccinimide as raw materials, and dissolve the above-mentioned raw materials in N, N-dimethylformamide was stirred at room temperature for 10 hours, and a large amount of solid was precipitated by adding water. The solid was washed and dried to obtain the corresponding bromide with a yield of 80%.
[0106] The second step: under the protection of nitrogen, put the product obtained in the first step and 4-(9-carbazolyl)phenylboronic acid (1:1 in molar ratio) into a two-necked bottle, and add a catalytic amount of tetrakis(triphenyl) ) phosphine palladium) and mixed solvent (sodium carbonate solution: toluene: ethanol = 1: 4: 4), keep the temperature at 90-100 ° C, stir for 24 hours, extract with ether, and the product is subjected to column chromatography (e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum brightness | aaaaa | aaaaa |
| Luminous efficiency | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com